Leveraging ChemAIRS to Investigate Synthetic Strategies for Tetrahydrobenzoazepine Core of BTK Inhibitor BIIB091_EP13
Overcoming Synthetic Challenges in BIIB091
BIIB091 is a potent, reversible inhibitor of Bruton’s tyrosine kinase (BTK), developed by Biogen as a potential small-molecule therapy for multiple sclerosis (MS). A key synthetic challenge in constructing BIIB091 lies in the preparation of its tetrahydrobenzoazepine fragment, which features a substituted tetrahydrobenzoazepine core and a chiral benzylic amine moiety—both requiring precise stereochemical control and advanced synthetic methodologies.
References: https://pubs.acs.org/doi/10.1021/acs.oprd.3c00133?ref=pdf
Synthetic Strategies for Tetrahydrobenzoazepine Core of BTK Inhibitor BIIB091
ChemAIRS explored multiple synthetic routes (Schemes 1–4) to the target molecule, emphasizing strategies for constructing the benzazepine core of N-Boc ketone.
Interested in exploring the capabilities of our Retrosynthesis module? Learn more here: ChemAIRS_Retrosynthesis
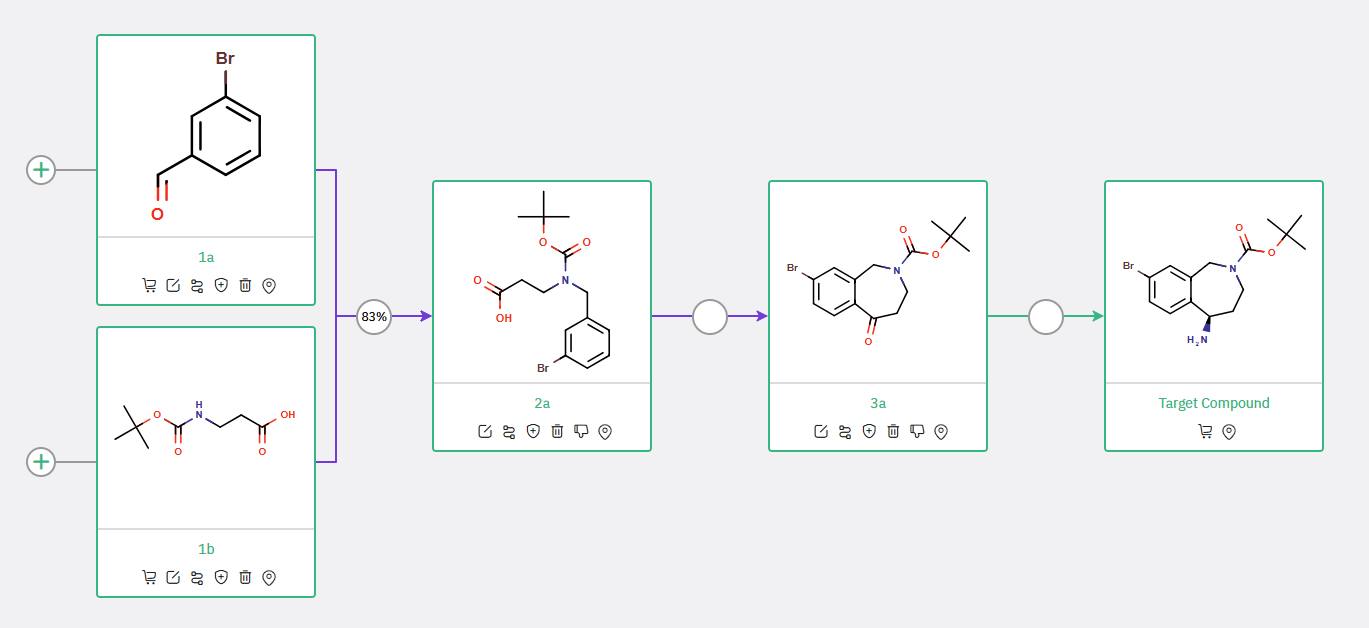
Scheme 1: Proposed synthetic route for Tetrahydrobenzoazepine, featuring Friedel-Crafts acylation to construct the benzazepine core from an N-Boc ketone
Scheme 2: Proposed synthetic route for Tetrahydrobenzoazepine, featuring Friedel-Crafts acylation to construct the benzazepine core from an N-Boc ketone
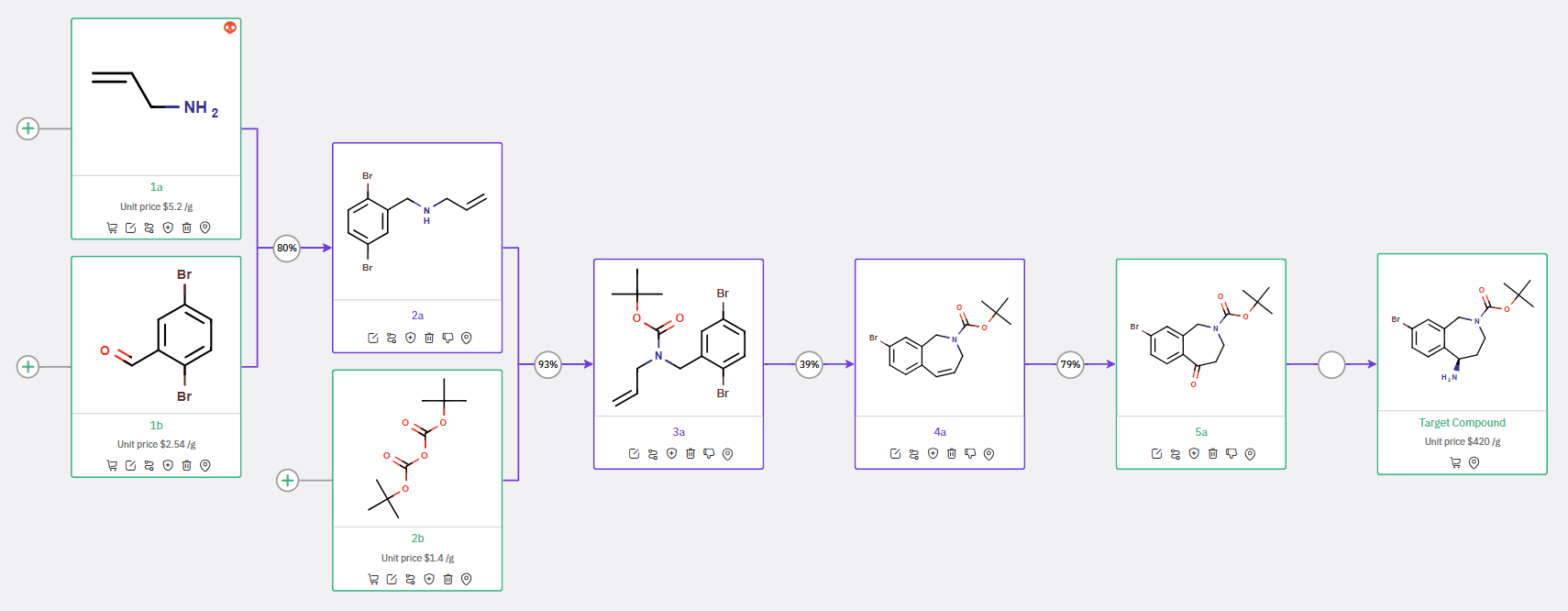
Scheme 3: Proposed synthetic route for Tetrahydrobenzoazepine, featuring Intramolecular Pd-Catalyzed Heck Reaction to construct the benzazepine core from an N-Boc ketone
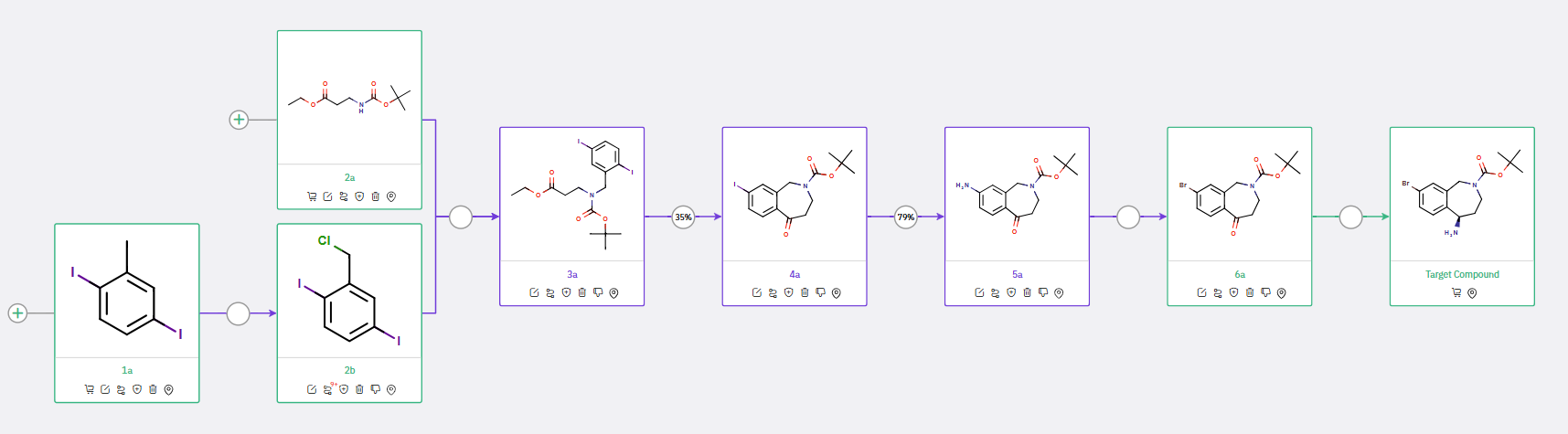
Scheme 4: Proposed synthetic route for Tetrahydrobenzoazepine, featuring Ester-Aryl Halide Cyclization to construct the benzazepine core from an N-Boc ketone
In the final step, in addition to the biocatalytic transamination of the N-Boc ketone using amine transaminase (ATA) as described in Biogen's published work, ChemAIRS proposed an alternative reductive amination protocol employing NH₄OAc/NaBH₃CN in iPrOH, as illustrated in Figure 1.
Figure 1: Alternative Reaction Condition for Synthesizing the Final Molecule
To achieve the key benzazepine framework, ChemAIRS identified three distinct synthetic strategies:
1.Friedel-Crafts (F-C) Acylation (Schemes 1 and 2):
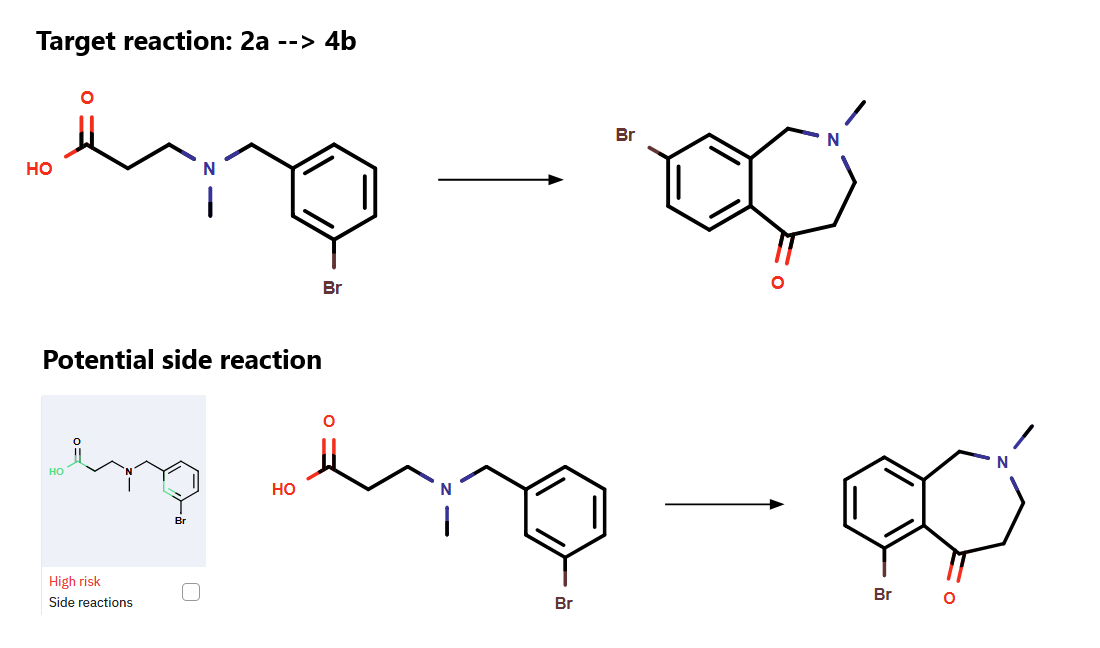
The F-C acylation served as the critical ring-forming step, though it proved challenging, with modest yields reported by Biogen. ChemAIRS proposed optimized reaction conditions to improve this transformation (Figure 2). Additionally, the algorithm flagged potential side reactions that could compromise the cyclization efficiency (Figure 3), providing insights into reaction optimization.
Figure 2: Alternative Reaction Conditions for Friedel-Crafts Acylation
Figure 3: Potential Side Reaction During Friedel-Crafts Acylation
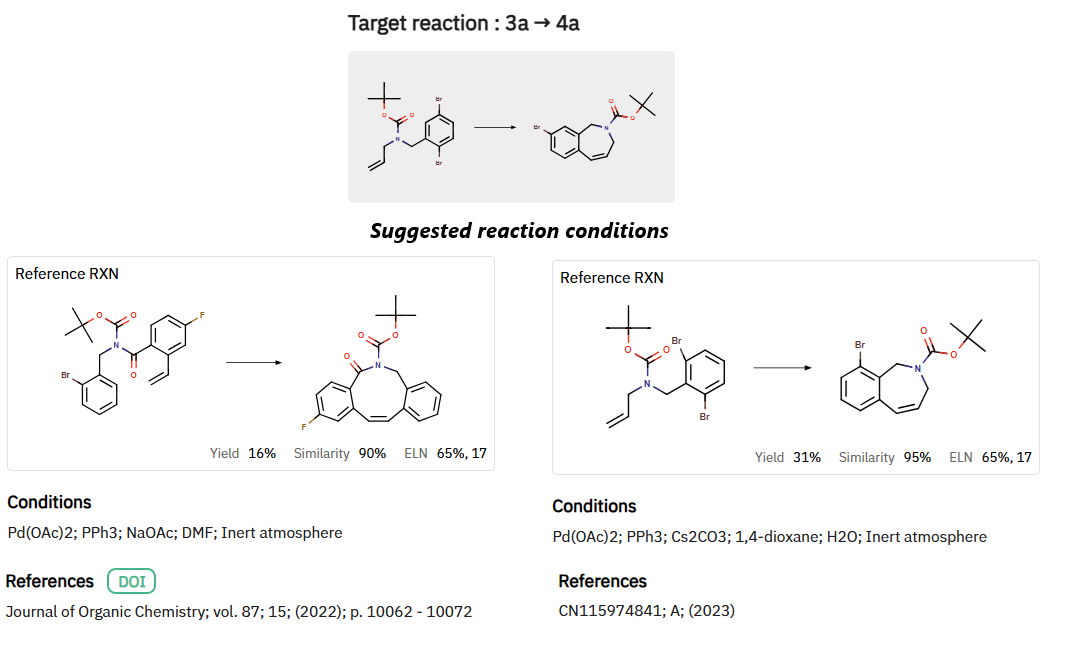
2.Intramolecular Pd-Catalyzed Heck Reaction (Scheme 3):
ChemAIRS proposed an alternative cyclization via an intramolecular Heck reaction, utilizing Pd catalysts. The system also identified a range of catalyst options to fine-tune this transformation for improved yield and selectivity (Figure 4).
Figure 4: Alternative Reaction Conditions for Intramolecular Pd-Catalyzed Heck Reaction
3.Ester-Aryl Halide Cyclization (Scheme 4):
A third approach involved cyclization through the reaction of a corresponding ester with an aryl iodide, offering another viable strategy for the formation of the benzazepine core.
By combining computational insights with chemical expertise, ChemAIRS highlighted these pathways as viable synthetic strategies, offering flexibility in addressing the challenges of benzazepine core construction.