Utilizing ChemAIRS to Investigate Synthesis Strategies for AZD5462: A Promising RXFP1 Agonist for Heart Failure Treatment_EP12
AZD5462, a small-molecule mimetic of relaxin H2 signaling at RXFP1, offering promise as a treatment for heart failure
AZD5462: A Promising RXFP1 Modulator for Heart Failure Treatment
Heart failure (HF) affecting an estimated 64 million people globally, arises from conditions such as hypertension, cardiomyopathy, coronary artery disease, and arrhythmias. Relaxin, a pleiotropic hormone, exhibits anti-fibrotic effects and promotes systemic and renal adaptations, with potential therapeutic benefits in HF, particularly acute decompensated heart failure (ADHF). Relaxin H2, a potent RXFP1 agonist, has shown promise in treating hypertension, kidney disease, and HF.
There remains a need for novel RXFP1 modulators with therapeutic potential. To address this, AstraZeneca and Mitsubishi Tanabe Pharma have developed AZD5462, a small-molecule mimetic of relaxin H2 signaling at RXFP1, offering promise as a treatment for heart failure.
References:
https://pubs.acs.org/doi/10.1021/acs.jmedchem.3c02184?ref=PDF
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2022122773
Optimizing Synthetic Pathways for AZD5462: ChemAIRS' Predictive and Scalable Solutions
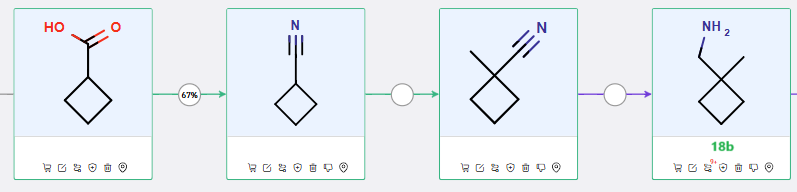
The 19-step synthetic route generated by ChemAIRS (Scheme 1) closely aligns the strategy outlined by AstraZeneca in 2022 (WO2022/122773), utilizing four key intermediates: 14a, 14b, 4b, and 18b. Notably, ChemAIRS proposed efficient synthetic routes for preparing one of the critical building blocks, 18b (Schemes 2), eliminating the need for commercial procurement.
Scheme 1: ChemAIRS predicted a synthetic pathway for AZD5462
Scheme 2: Proposed synthetic route for intermediate 18b
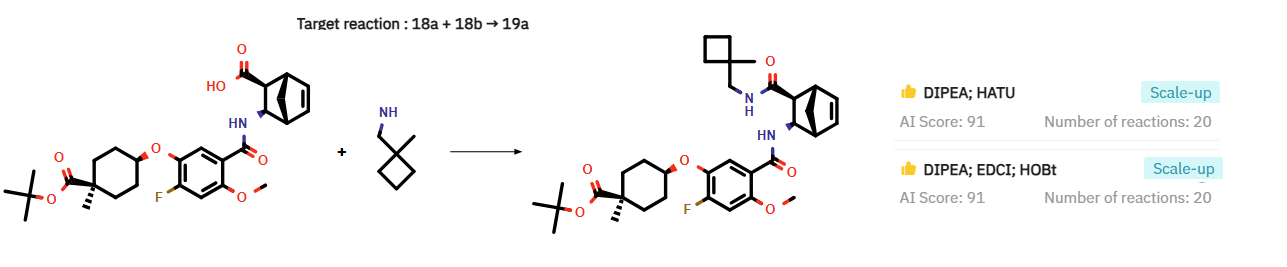
In addition to replicating the reaction conditions specified in the AstraZeneca patent for the amide coupling to synthesize 19a (DMF, DIPEA, HATU), ChemAIRS identified an alternative "scale-up friendly" protocol (Figure 1).
Figure 1: Proposed alternative scale-up conditions for intermediate 19a
While the literature method does not address potential side reactions, ChemAIRS detected a high-risk side reaction associated with the synthesis of 19a (Figure 2). This predictive capability provides chemists with advanced warning and supports the development of robust contingency strategies.
Figure 2: Possible side reaction in the synthesis of intermediate 19a
Alternative Synthetic Approach to AZD5462
Scheme 3 illustrates an alternative synthetic strategy for the preparation of AZD5462, in which ChemAIRS proposes modified approaches for constructing the key intermediates, 7a and 7b. Notably, these routes reduce the overall step count compared to the previously described pathway in Scheme 1. Specifically, the two-step synthesis of 7b begins with an oxidative hydroxylation of arylboronic acid 1a, followed by a coupling reaction with benzyl alcohol. Furthermore, unlike the sequence presented in Scheme 1, ChemAIRS recommends performing the amide coupling as the final step. This adjustment aims to mitigate the risk of the side reaction depicted in Figure 2.
Scheme 3: An alternative synthetic strategy to obtain AZD5462
In summary, ChemAIRS empowers chemists to rapidly optimize synthesis strategies by efficiently proposing synthetic routes, identifying potential side reactions, and suggesting scalable alternative conditions. Its advanced predictive capabilities, including risk assessment for critical reactions, enable researchers to proactively address challenges in route development while minimizing reliance on external resources.
Interested in exploring the capabilities of our Process Chemistry module? Learn more here: ChemAIRS_Process Chemistry