Leveraging ChemAIRS to Investigate Synthetic Approaches for Azetidine-Based αvβ6 Integrin Inhibitors: Core Modifications of GSK3335103_EP17
Targeting αvβ6 Integrin: Novel Oral Inhibitors for Fibrotic Disease Therapy
In recent years, integrin proteins have emerged as promising therapeutic targets for a range of diseases, including idiopathic pulmonary fibrosis (IPF). Among these, the RGD integrins—a subfamily that binds to the arginine-glycine-aspartate (RGD) motif of endogenous ligands—are particularly notable. Several RGD integrins, including αvβ6, play a critical role in activating transforming growth factor beta 1 (TGF-β1), a key mediator of fibrogenesis and a central player in the pathogenesis of fibrosis. The αvβ6 integrin has been specifically identified as a compelling target for the treatment of fibrotic diseases due to its role in TGF-β1 activation.
GSK recently reported the design and development of a novel series of orally bioavailable αvβ6 inhibitors, building on their earlier compounds GSK3008348 and GSK3335103. Two lead compounds from this series, (S)-20 and 28, exhibited favorable oral pharmacokinetic profiles in rats, suggesting their potential as orally bioavailable therapeutic agents for IPF.
References: https://pubs.acs.org/doi/10.1021/acs.jmedchem.4c02051?ref=PDF
ChemAIRS Revolutionizes Lead Molecule Synthesis with Optimized Routes
ChemAIRS played a critical role in streamlining the synthetic routes for these lead molecules by optimizing reaction sequences and addressing challenges associated with intermediates. For compound 20, ChemAIRS replaced a labor-intensive Wittig reaction with a more efficient Horner–Wadsworth–Emmons reaction, enhancing scalability and selectivity while minimizing side reactions. For compound 28, ChemAIRS proposed a synthetic route leveraging palladium-catalyzed cross-coupling and asymmetric Friedel–Crafts alkylation, enabling the production of a high-purity, enantiomerically enriched product.
ChemAIRS-Proposed Synthetic Strategy for Compound (S)-20
The synthetic approach proposed by ChemAIRS is illustrated in Scheme 1, beginning with the commercially available tert-butyl 3-(2-oxoethyl)azetidine-1-carboxylate (3a), consistent with a method reported by GSK. Oxidation of aldehyde 3a with N-fluorobenzenesulfonimide (NFSI) yielded difluoroaldehyde 4a, which was subsequently subjected to a Horner–Wadsworth–Emmons (HWE) reaction with the diphosphorylated intermediate 4b, affording the target alkene 5a.
Scheme 1: ChemAIRS-Proposed Synthetic Strategy for Compound (S)-20 in GSK's publication
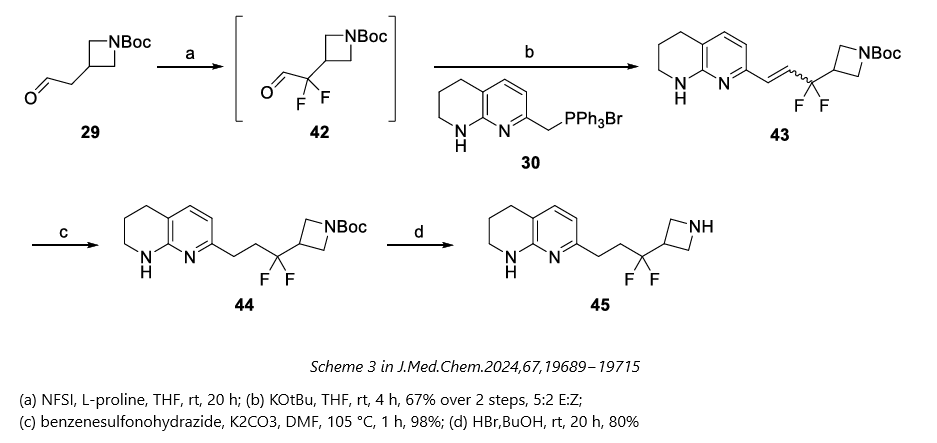
GSK previously described a route to difluoroazetidine 45 via a Wittig reaction between phosphonium salt 30 and aldehyde 42 (Figure 1). However, the Wittig approach involved a six-step synthesis of phosphonium salt 30 and encountered challenges such as byproduct formation and selectivity issues.
Figure 1: Wittig Reaction-Driven Synthesis of a Difluoroazetidine Intermediate
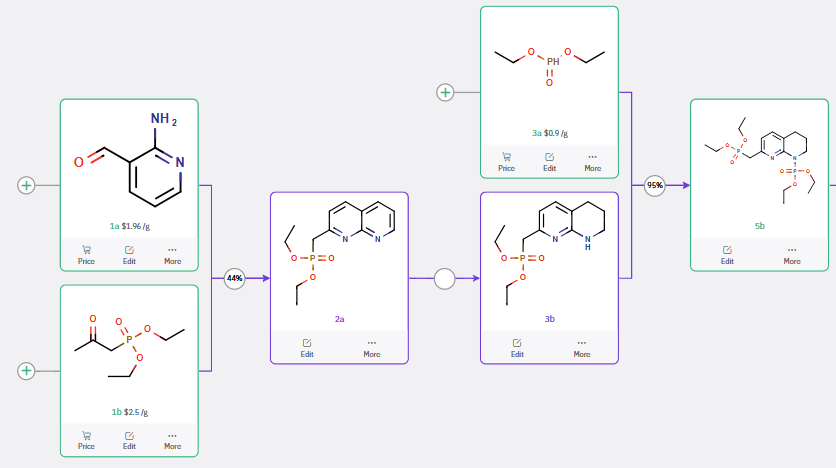
In contrast, the HWE reaction demonstrated high yields and could offer advantages in scalability, as referenced in Figure 2.
Figure 2: Horner–Wadsworth–Emmons Reaction for the Synthesis of a Fluoroazetidine Intermediate
The conjugate addition of arylboronic acid 8b to 4-oxoisocrotonic acid 8a, catalyzed by a chiral diene/Rh(I) complex, afforded compound 9a with excellent enantioselectivity. However, ChemAIRS flagged a potential high-risk side reaction at this step (Figure 3). The subsequent reductive amination between 9a and 9b successfully produced the desired target molecule.
Figure 3: ChemAIRS Identified a Potential Side Reaction in the Synthesis of Intermediate 9a
Considering the hazardous nature of diethyl chlorophosphate (1a and 2a in Scheme 1) utilized for synthesizing the diphosphorylated intermediate 4b, ChemAIRS recommended an alternative synthetic pathway for the bisphosphonate, as detailed in Scheme 2.
Scheme 2: Alternative pathway to synthesize diphosphorylated intermediate
ChemAIRS-Proposed Synthetic Strategy for Compound 28
Another promising lead compound, 28, highlighted in GSK’s publication, is identified as a potent and orally bioavailable αvβ6 inhibitor with a difluoromethylazetidine core. ChemAIRS proposed a synthetic route to access this lead molecule (Scheme 3). The synthesis commenced with a palladium-catalyzed cross-coupling reaction between intermediates 3a and 3b, yielding compound 4a, followed by Boc-deprotection and fluorination to afford difluoroazetidine 8b.
Scheme 3: ChemAIRS-Proposed Synthetic Strategy for Compound 28 in GSK's publication
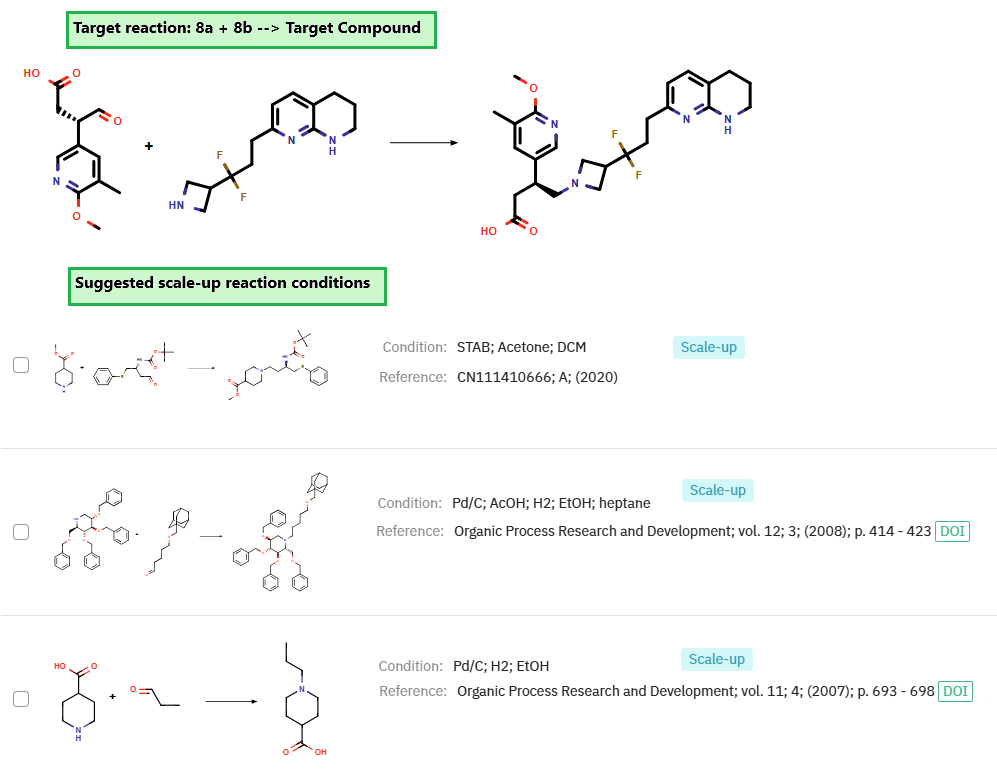
The second phase of the synthesis involved an asymmetric Friedel–Crafts alkylation between pyridine 6a and maleic acid monoethyl ester 6b, generating intermediate 7a, which was subsequently reduced to provide compound 8a. The final step, a reductive amination between 8a and 8b, furnishes the target molecule. ChemAIRS also recommended optimized conditions for scaling up this final step (Figure 4).
Interested in exploring the capabilities of our Process Chemistry module? Learn more here: ChemAIRS_Process Chemistry
Figure 4: ChemAIRS Proposed Optimized Scale-Up Conditions for the Final Step
The lead compound 28, as reported in the GSK study, was presumed to be an enantioenriched mixture with the (S)-enantiomer as the predominant species. By employing the strategy suggested by ChemAIRS, it is anticipated that synthesis can achieve a product with high optical purity.
ChemAIRS provides substantial benefits in designing efficient and scalable synthetic strategies for complex molecules, streamlining reaction pathways, and overcoming challenges associated with intermediates. The synthesis of two lead molecules, identified as potent and orally bioavailable αvβ6 inhibitors, highlights the utility of ChemAIRS in retrosynthetic planning. By enabling innovative and practical solutions, ChemAIRS plays a pivotal role in expediting the development of lead compounds in medicinal chemistry.