Utilizing ChemAIRS to Explore Synthetic Strategies for BAY 2413555: A Bayer Therapeutic Candidate Targeting Heart Failure_EP16
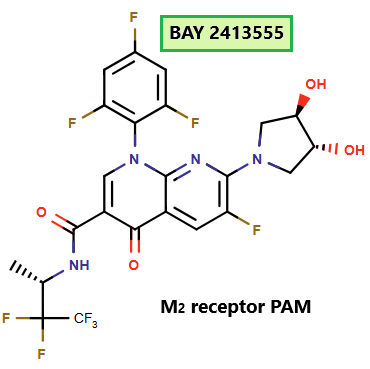
BAY 2413555: A Selective M2 Receptor Modulator for Heart Failure – Promise and Clinical Setback
Heart failure (HF) with reduced ejection fraction involves autonomic imbalance, with increased sympathetic and reduced parasympathetic activity. BAY 2413555, a selective and reversible M₂ receptor (M2R) positive allosteric modulator (PAM), was developed to enhance parasympathetic signaling and restore cardiac autonomic balance. Identified through preclinical studies, it advanced to clinical trials but was terminated in March 2024 due to vascular inflammation observed in animals.
Before termination, BAY 2413555 showed good safety and tolerability, with no serious adverse events. Preclinical and clinical data highlighted its potential to improve heart rate and variability, supporting its therapeutic promise for HF.
Reference: https://pubs.acs.org/doi/10.1021/acs.jmedchem.4c01590?ref=PDF
ChemAIRS Proposes Retrosynthetic Strategy for BAY 2413555: Key Disconnections and Synthetic Pathways
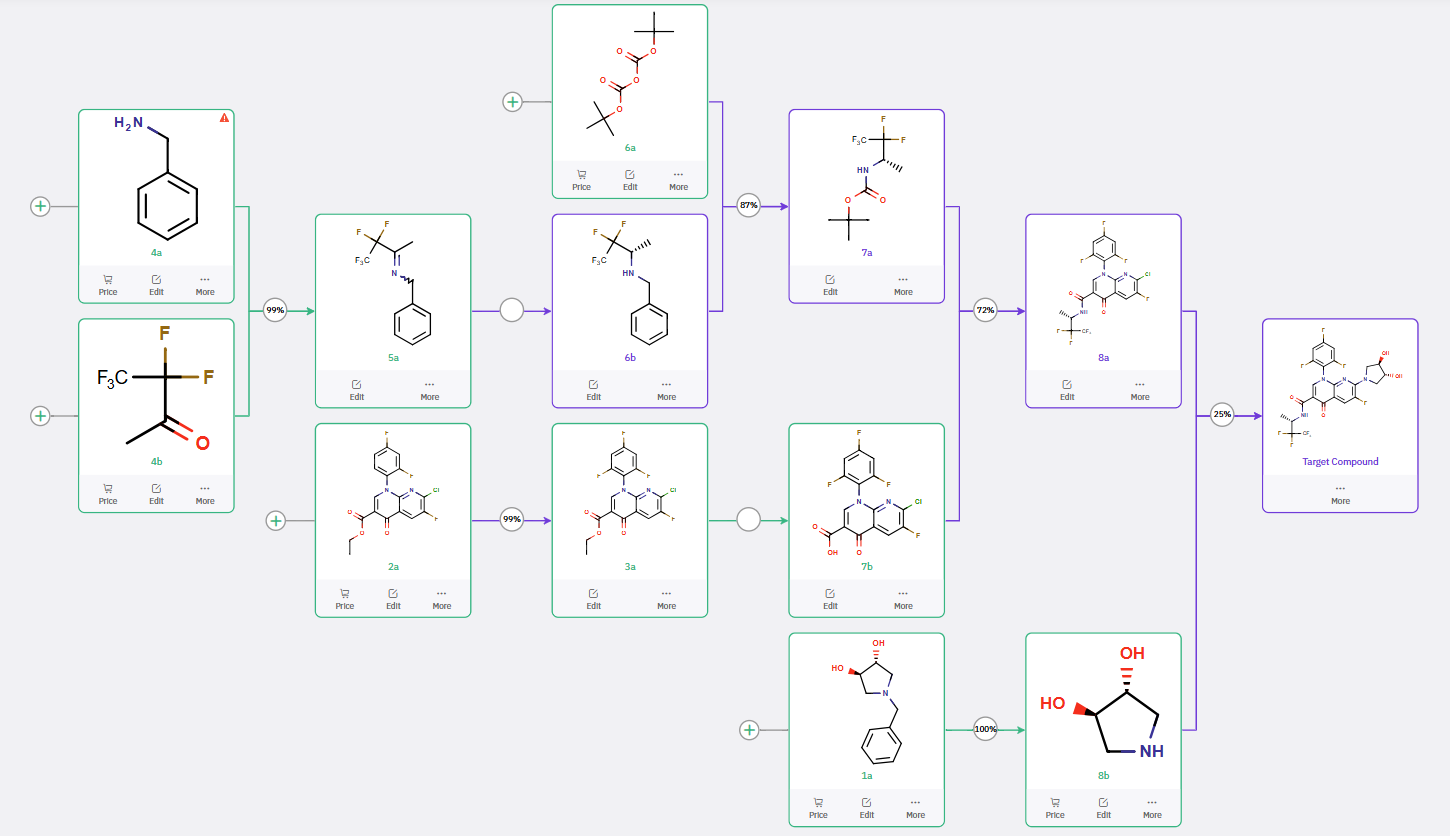
ChemAIRS generated a retrosynthetic blueprint for BAY 2413555, identifying a key disconnection strategy that involves three key building blocks: naphthyridine carboxylates 4a, dihydroxypyrrolidine 4b, and pentafluoroalkyl amine 6a (Scheme 1). The proposed pathway initiates with an SnAr reaction between 4a and 4b, followed by ester hydrolysis to furnish intermediate 6b. The synthesis of pentafluoroalkyl amine 6a proceeds via a transamination of commercially available precursors 5a and 5b, with chiral chromatographic separation potentially required to isolate the desired stereoisomer. The final step involves an amide bond formation, where HATU serves as the coupling reagent, with ChemAIRS also suggesting alternative activation conditions utilizing HBTU/DIPEA or NMM/IBCF.
Scheme 1: ChemAIRS-Proposed Retrosynthetic Strategy for BAY 2413555
To enhance route flexibility, ChemAIRS proposed additional strategies for accessing the pentafluoroalkyl amine intermediate (Scheme 2). One alternative involves direct asymmetric reductive amination (route 2a), while another employs a sulfinamide intermediate, which undergoes subsequent deprotection to yield the target amine (route 2b).
Scheme 2: ChemAIRS-Proposed Additional Atrategies for the Pentafluoroalkyl Amine intermediate
ChemAIRS-Suggested Alternative Pathway to BAY 2413555 with Scalable Reaction Conditions
A divergent synthetic approach was outlined in Scheme 3, where amide formation was strategically shifted earlier in the sequence. Instead of performing amide coupling at the final stage, intermediate 8a underwent an SnAr reaction with amine 8b to directly construct the target molecule.
Scheme 3: ChemAIRS-Suggested Alternative Pathway to BAY 2413555
Additionally, ChemAIRS provided scale-up recommendations for the final amidation step, offering base choices such as DIPEA, TEA, or K₂CO₃ to optimize reaction efficiency under large-scale conditions (Figure 1).
Interested in exploring the capabilities of our Process Chemistry module? Learn more here: ChemAIRS_Process Chemistry
Figure 1: ChemAIRS Proposed Optimized Scale-Up Conditions for the Final Step
ChemAIRS demonstrated its versatility in retrosynthetic planning by proposing multiple synthetic routes to BAY 2413555, offering chemists various options to optimize their workflows. The AI-powered tool not only identified key disconnections but also suggested alternative strategies for critical intermediates, such as pentafluoroalkyl amines, and provided scalable reaction conditions. By integrating AI-driven retrosynthetic analysis with practical considerations like reagent selection and scalability, ChemAIRS enhances efficiency in synthetic route design, empowering chemists with data-driven decision-making for complex molecule synthesis.