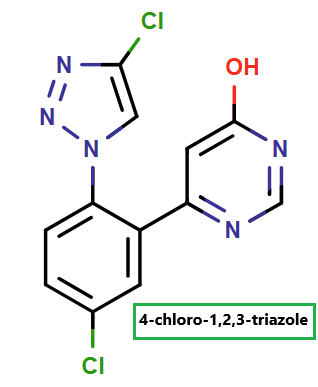
Enhancing Synthetic Pathways and Tackling Impurities: How ChemAIRS Supports the Synthesis of 4-Chloro-1,2,3-Triazole for Milvexian_EP15
Milvexian: An Innovative FXIa Inhibitor for Thrombosis Prevention
Cardiovascular diseases (CVDs) remain a leading global cause of mortality, demanding breakthrough therapies. Inhibiting Factor XIa (FXIa) represents a promising therapeutic strategy to mitigate vascular and thromboembolic diseases while maintaining normal hemostasis. Milvexian (BMS-986177/JNJ 70033093) is a first-in-class oral FXIa inhibitor, and offers promising solutions. With its potential to prevent thrombotic events without compromising normal hemostasis, Milvexian is poised to become a game-changer in vascular health.
To support the development of Milvexian, Bristol Myers Squibb and Janssen Pharmaceuticals have jointly advanced scalable synthetic methodologies for 4-chloro-1,2,3-triazole, a critical intermediate in its production.
Reference: https://pubs.acs.org/doi/10.1021/acs.oprd.2c00399?ref=pdf
ChemAIRS' Discovery Chemistry Approaches for 4-Chloro-1,2,3-Triazole
ChemAIRS proposed multiple synthetic pathways for the preparation of 4-chloro-1,2,3-triazole, an essential intermediate in the synthesis of Milvexian. The proposed routes utilized diverse commercially available starting materials, with two representative strategies detailed below:
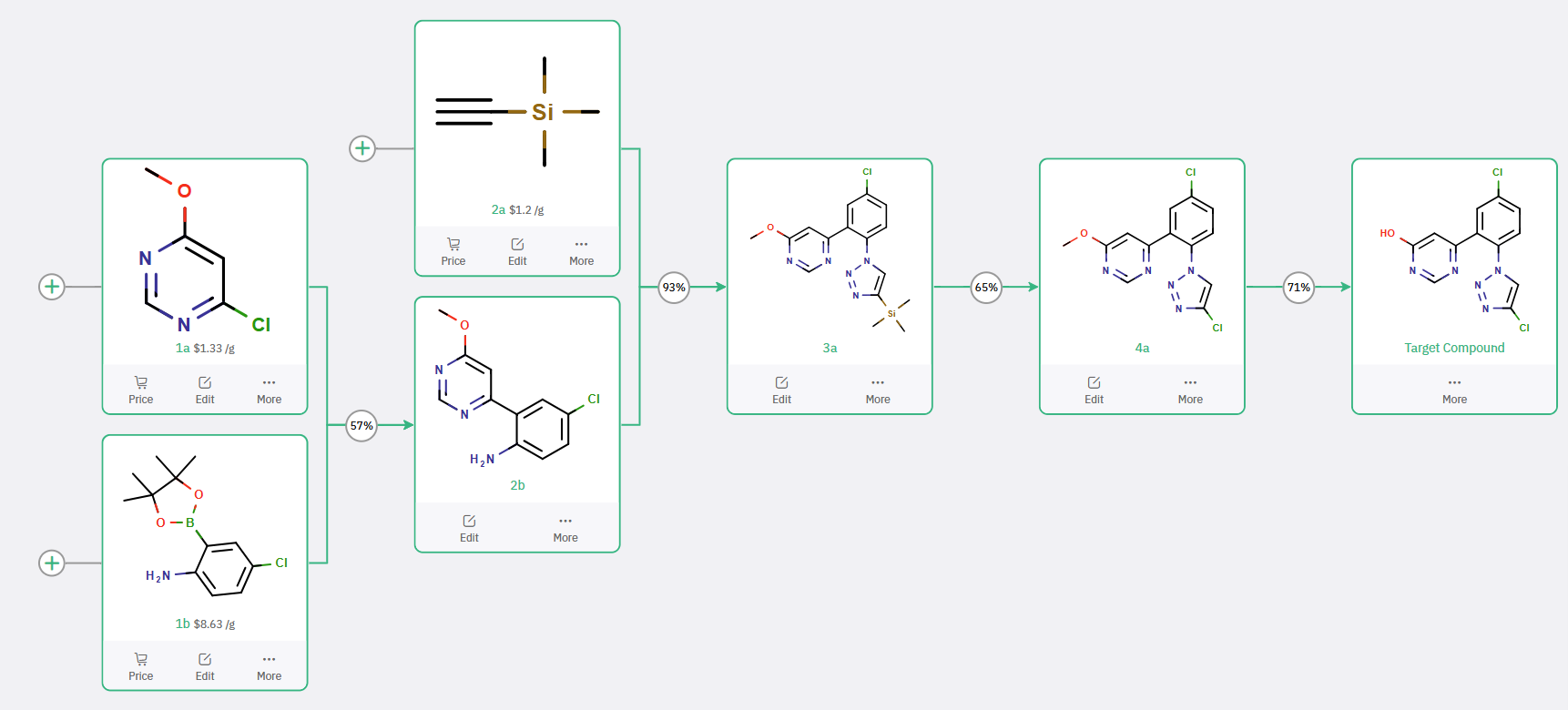
Scheme 1: ChemAIRS Predicts a Synthetic Pathway for 4-Chloro-1,2,3-Triazole Aligned with Bristol Myers Squibb's Approach
The synthetic approach in Scheme 1 closely parallels the discovery route reported by Bristol Myers Squibb. In this route, boronic ester 1b undergoes a Suzuki−Miyaura coupling with 4-chloro-6-methoxypyrimidine 1a, yielding aniline 2b. This intermediate is subsequently transformed into 4-TMS-substituted triazole 3a through a sequence of diazotization/azidation followed by regioselective click chemistry. A chlorination step replaces the trimethylsilyl (TMS) group with a chlorine atom, and O-demethylation affords the desired product, 4-chloro-1,2,3-triazole.
An alternative synthetic route (Scheme 2) involves the direct conversion of TMS-substituted triazole 2a into aryl triazole 3a via a single-step reaction. This method, reported by Janssen Pharmaceuticals, employs a ruthenium(II) carboxylate complex in conjunction with an electron-deficient phosphine ligand to achieve efficient transformation.
Scheme 2: Discover ChemAIRS' proposed synthetic route for 4-Chloro-1,2,3-Triazole, highlighting triazole-enabled ruthenium-catalyzed C–H arylations
The simplicity of this route offers a user-friendly approach, particularly attractive for drug development. However, ChemAIRS identified high-risk alerts associated with potential side reactions (Figure 1), which may have contributed to the moderate yield (~50%) observed in this process. These findings underscore the challenges of optimizing such transformations for practical scalability.
Figure 1: ChemAIRS identifies potential side reactions as risk items for streamlined synthesis
Impurity Prediction: A Crucial Element in Scale-Up Synthesis
The synthetic route reported by Bristol Myers Squibb demonstrated acceptable yields, product quality, and reproducibility at the laboratory scale. However, it proved insufficient for plant-scale production to meet clinical supply demands, primarily due to the formation of impurities as side products during the synthesis.
In the Suzuki−Miyaura coupling step, in addition to the desired product, an undesired Buchwald−Hartwig impurity (compound 11) was observed. This impurity arises from competing C−N coupling between intermediates 3 and 4 (Figure 2).
Figure 2: Impurity identified in the Suzuki-Miyaura coupling step, as reported by Bristol Myers Squibb
To evaluate the impurity profile, ChemAIRS’ Impurity Prediction module was employed. By inputting comprehensive reaction data—including the reaction scheme, exact mass of the impurity, and details of the solvents, reagents, and catalysts—the module predicted over 30 potential impurity structures, each assigned a likelihood score ranging from 0 to 100. Notably, one of the predicted structures, with a score of 100, perfectly matched the impurity reported by Bristol Myers Squibb (Figure 3).
Figure 3: ChemAIRS predicts impurity structures for the Suzuki-Miyaura coupling step
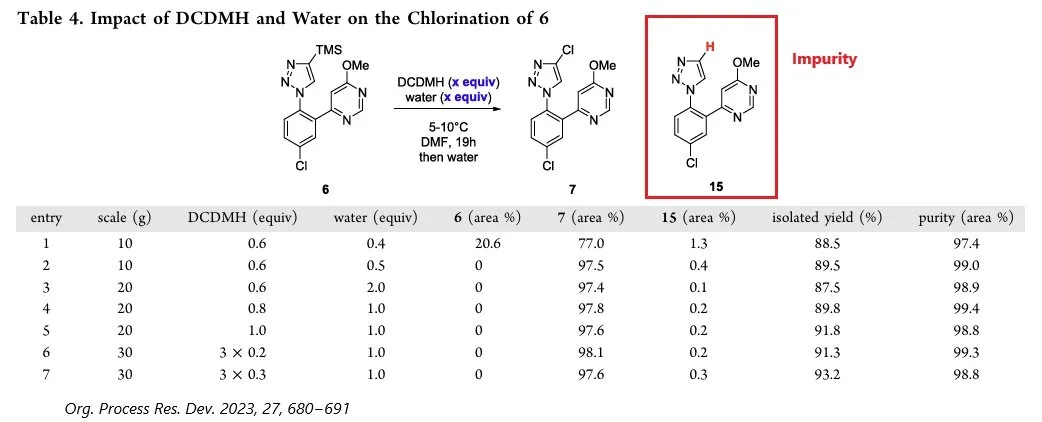
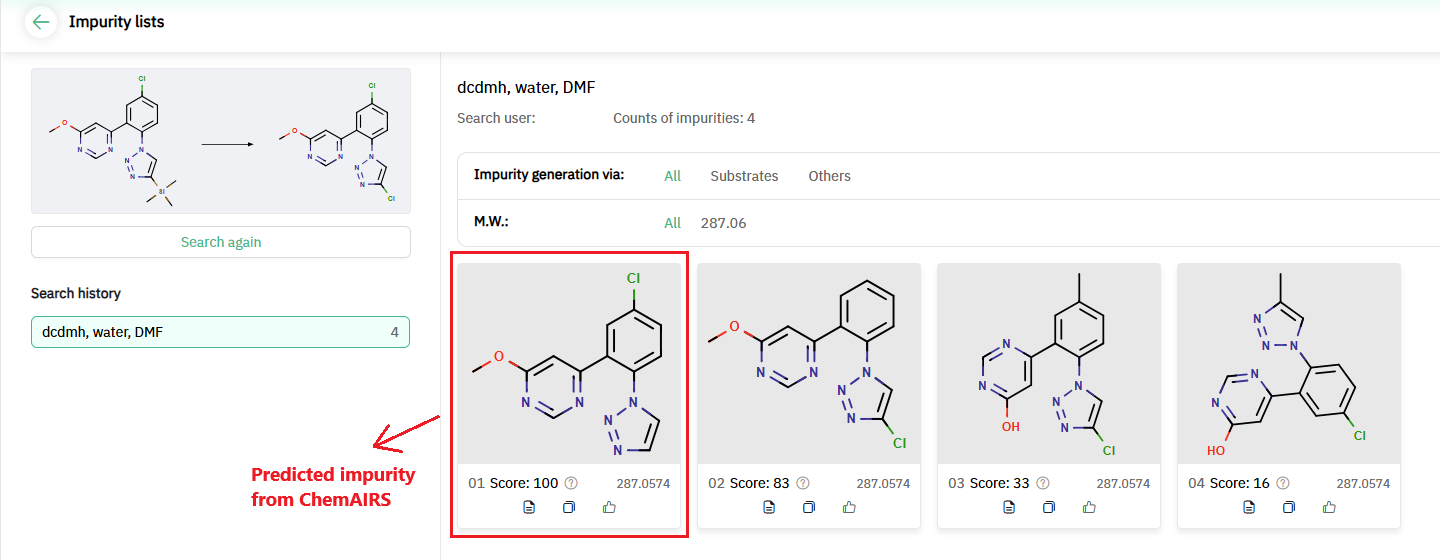
Encouraged by this result, we extended the impurity analysis to the chlorination of 4-TMS-substituted triazole. According to published data, impurity 15 forms via cleavage of the carbon-silicon bond in compound 6 during the chlorination step (Figure 4). Using the Impurity Prediction module, ChemAIRS identified four possible structures for impurities, one of which, with the highest likelihood score (score 100), corresponded to the impurity described by Bristol Myers Squibb (Figure 5).
Interested in exploring the capabilities of our Impurity Prediction module? Learn more here: ChemAIRS_Impurity Prediction
Figure 4: Impurity identified in the chlorination step, as reported by Bristol Myers Squibb
Figure 5: ChemAIRS predicts impurity structures for the chlorination step
ChemAIRS exemplifies the power of AI in tackling the complexities of modern drug discovery. Its ability to optimize synthetic pathways, predict impurities, and address scale-up challenges makes it an essential partner for researchers aiming to bring groundbreaking therapies like Milvexian to life.