Using ChemAIRS to Accelerate Discovery of Synthetic Strategies for KT474: A Selective, Orally Bioavailable Heterobifunctional IRAK4 Degrader from Kymera Therapeutics_EP14
KT-474: A First-in-Class IRAK4 Degrader Advancing Autoimmune and Inflammatory Disease Therapies
Interleukin-1 receptor-associated kinase 4 (IRAK4) plays a critical role in the IL-1R and TLR signaling pathways, which are associated with numerous autoimmune and inflammatory conditions. Targeting IRAK4 through degradation has emerged as a promising strategy for addressing these diseases.
Kymera Therapeutics has developed KT-474 (SAR444656), a first-in-class, selective, and orally bioavailable heterobifunctional IRAK4 degrader. In collaboration with Sanofi, KT-474 is being evaluated in double-blind, randomized, placebo-controlled Phase 2 clinical trials for hidradenitis suppurativa (HS) and atopic dermatitis (AD).
References: https://pubs.acs.org/doi/10.1021/acs.jmedchem.4c01305?ref=PDF
Optimizing Synthetic Pathways for KT-474: ChemAIRS' Predictive and Scalable Solutions
The synthetic approach to KT-474 devised by ChemAIRS consists of a 13-step sequence (Scheme 1), which closely parallels the synthetic pathway previously reported by Kymera Therapeutics. This synthetic design revolves around three key intermediates: 11a, 11b, and 13b. Notably, 11b was efficiently prepared in a single step from precursor 9b, mirroring the simplicity of Kymera's methodology. The synthesis of 13b required an eight-step sequence, consistent with Kymera's reported strategy.
Scheme 1: ChemAIRS predicted a synthetic pathway for KT-474
However, a significant divergence lies in the preparation of 11a: ChemAIRS developed a streamlined one-step synthesis from commercially available precursors 10a and 10b, a marked improvement over the six-step route outlined by Kymera, offering notable savings in both time and labor.
The final assembly of KT-474 was accomplished via a sequence of transformations. First, 11a underwent a Sonogashira coupling with 11b, followed by deprotection to yield intermediate 13a. This intermediate was then coupled with 13b to furnish KT-474. ChemAIRS also proposed an alternative scale-up protocol for the final step, utilizing STAB as the primary reducing agent (Figure 1), in contrast to Kymera's method involving HOAc/NaBH(OAc)3/TEA, potentially providing operational and scalability advantages.
Interested in exploring the capabilities of our Process Chemistry module? Learn more here: ChemAIRS_Process Chemistry
Figure 1: ChemAIRS proposed alternative reaction conditions to the target molecule
Alternative Synthetic Approach to KT-474
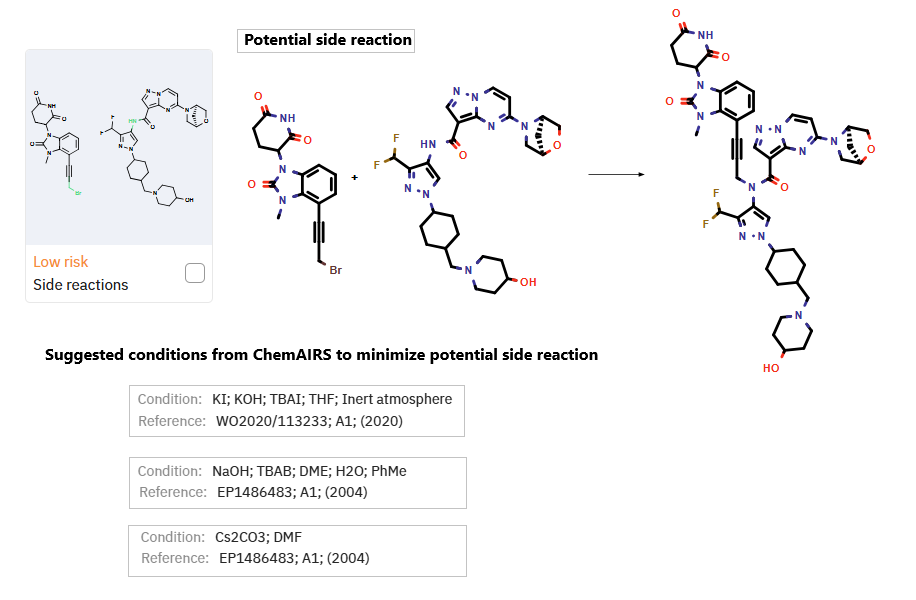
In a second synthetic route proposed by ChemAIRS (Scheme 2), the synthesis centers around four building blocks: 10a, 7a, 6a, and 6b. Amide coupling between 6a and 6b produced intermediate 7b, which subsequently reacted with 7a to yield 11b. Meanwhile, bromination of 10a generated 11a, which then underwent coupling with 11b to afford KT-474. During this route, ChemAIRS identified a potential side reaction (Figure 2) in the final coupling step. To mitigate this, optimized reaction conditions were proposed, offering a refined strategy to minimize product loss and maximize yield.
ChemARIS Proposed Alternative synthetic pathway to KT-474
Scheme 2: ChemAIRS’ proposed alternative synthetic pathway to KT-474
Figure 2: ChemAIRS identified a potential side reaction in the final step and proposed optimized reaction conditions to minimize its occurrence.
The synthetic strategies for KT-474 proposed by ChemAIRS demonstrate notable advancements in efficiency and scalability, with simplified key steps like the one-step synthesis of 11a and alternative scale-up conditions enhancing the practicality of the process. Additionally, the proactive identification and mitigation of side reactions reflect a forward-thinking approach to process optimization, highlighting valuable contributions to synthetic organic chemistry and pharmaceutical development.