Optimizing Synthetic Strategies of Genentech's EPB inhibitor with ChemAIRS_EP02
Targeting EBP with Novel Brain-Penetrant Inhibitor Offers Promising Strategy for Multiple Sclerosis Remyelination Therapy
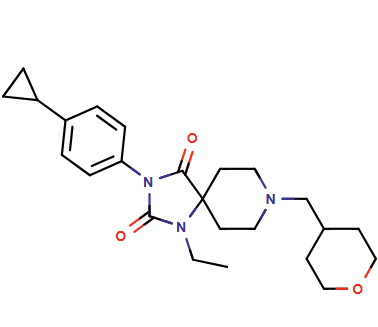
Targeting emopamil binding protein (EBP), a sterol isomerase in the cholesterol biosynthesis pathway, has been implicated in promoting oligodendrocyte differentiation, presenting a potential strategy for multiple sclerosis therapy. Earlier this year, Genentech and Convelo Therapeutics reported a novel, brain-penetrant, orally bioavailable EBP inhibitor, serving as a promising in vivo tool for evaluating remyelination therapies.
Reference: https://doi.org/10.1021/acs.jmedchem.3c02396
AI-Powered Retrosynthesis by ChemAIRS: Uncovering Innovative Synthetic Pathways for an EBP Inhibitor
Our retrosynthesis platform ChemAIRS predicted multiple unreported viable synthetic routes (shown in purple) that closely align with the reported strategies from Genentech and Convelo Therapeutics.
We selected the two most efficient synthetic pathways that start from two different sets of building blocks.
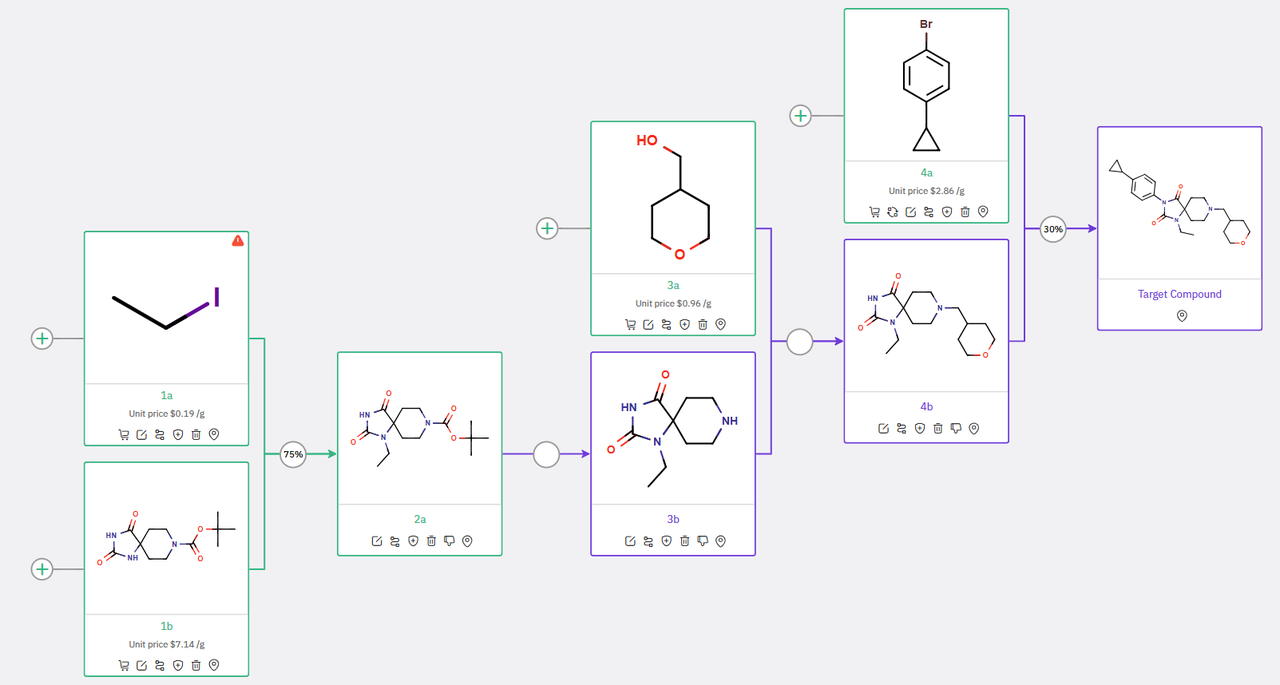
In the first proposed synthetic scheme (Scheme 1), commercially available spirohydantoin 1b undergoes selective monoalkylation with ethyl iodide to yield intermediate 2a. Subsequent N-Boc deprotection results in intermediate 3b, which is then subjected to intermolecular alkylation with hydroxymethyltetrahydropyran 3a to produce compound 4b. The final target molecule is obtained via a copper-mediated arylation between 4b and 1-bromo-4-cyclopropylbenzene.
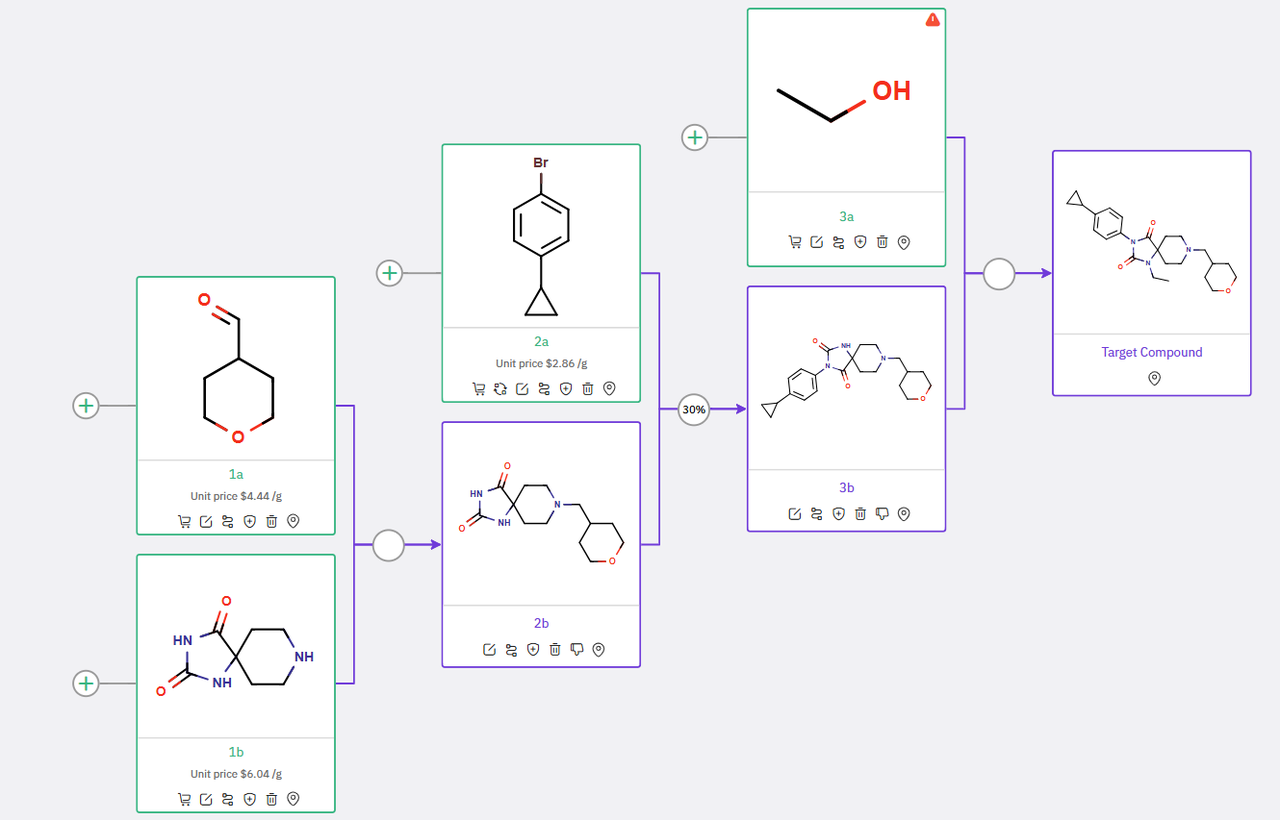
With a different set of starting materials, an alternative synthetic approach is detailed in Scheme 2. This pathway begins with a reductive-amination between spirohydantoin 1b and formyltetrahydropyran 1a, yielding intermediate 2b. This intermediate then undergoes copper-mediated arylation to form hydantoin compound 3b, which is subsequently converted to the final API via N-alkylation.
Scheme 1: ChemAIRS’ Proposed Synthetic Pathway to an EBP inhibitor
Scheme 2: ChemAIRS’ Proposed Alternative Synthetic Pathway to an EBP inhibitor, with a different set of starting materials
The results were generated within minutes, providing researchers with a range of viable options to efficiently synthesize de novo compounds based on building block availability and costs.