Utilizing ChemAIRS to investigate synthesis strategy of PRMT5 inhibitor from Gilead Sciences_EP01
PRMT5 as a Therapeutic Target in Cancer Epigenetics: Role in Arginine Methylation and Oncogenic Progression
Protein arginine methyltransferase 5 (PRMT5), the primary type II arginine methyltransferase, catalyzes the mono- and symmetric dimethylation of arginine residues in both histone and non-histone proteins. Recent evidence has shown that PRMT5 is crucial in the onset and progression of various human cancers by promoting cell proliferation, invasion, and migration. Consequently, PRMT5 has emerged as a promising and valuable target for cancer epigenetic therapy.
References: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2024137852&_cid=P11-LXXB1Y-93883-1
ChemAIRS Proposed Synthetic Strategies for PRMT5 inhibitor
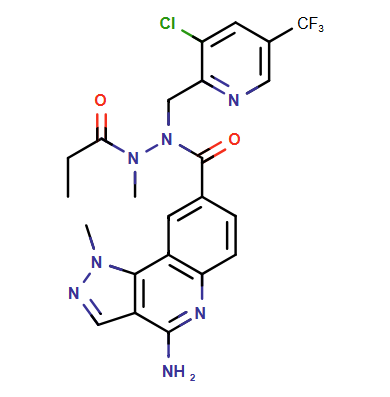
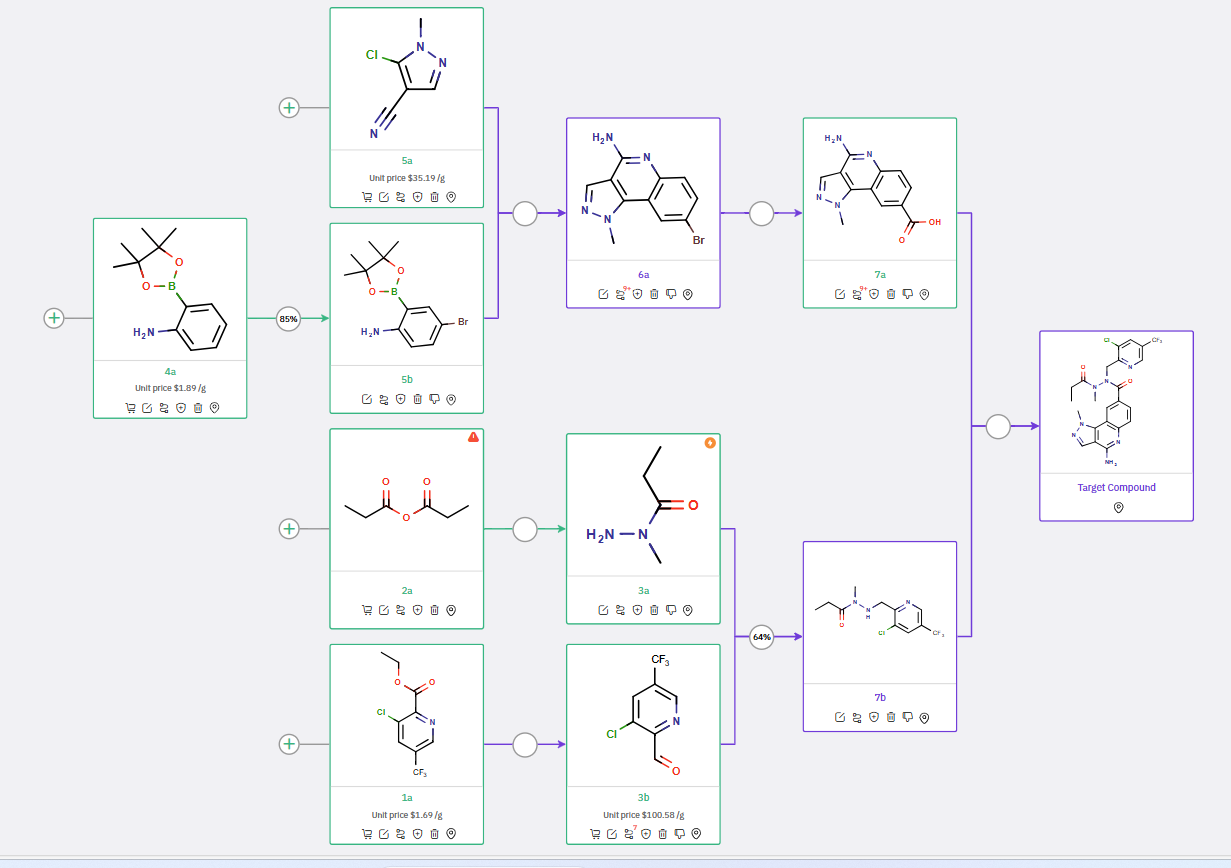
Within minutes, ChemAIRS identified a practical 7-step synthetic pathway that includes several unreported intermediates and predictive synthetic steps, as shown by the purple boxes and arrows in the scheme below. This strategy involves the synthesis of two key intermediates: pyrazoloquinoline carboxylic acid 7a and pyridyl-methylpropane hydrazide 7b.
ChemAIRS' Proposed synthetic Pathway for PRMT5 inhibitor
The first part of the procedure utilizes a Suzuki coupling reaction between amino boronic ester 5b (prepared via a bromination of 4a) and commercially available pyrazole carbonitrile 5a to yield pyrazoloquinoline 6a. Subsequent carboxylation of the aryl bromide 6a with carbon dioxide generates the carboxylic acid intermediate 7a.
In parallel, intermediate 7b is synthesized through a reductive amination reaction between hydrazide 3a and pyridinecarboxaldehyde 3b. The resultant methylpropanehydrazide 7b is then coupled with pyrazoloquinoline carboxylic acid 7a to deliver the final API.
Overall, the proposed synthetic route aligns closely with the latest work from Gilead Sciences, highlighting the reliability and efficiency of the ChemAIRS Retrosynthesis tool. Our innovative Retrosynthesis tool demonstrates its capability by suggesting realistic and unreported synthetic strategies for novel molecules, ensuring that researchers can explore new pathways with confidence.