Enabling Retrosynthetic Planning for Radiotheranostics: ChemAIRS-Driven Synthesis of PSMA and NTSR1 Ligands
Prostate Cancer and the Rise of Radiotheranostics
Prostate cancer remains the most frequently diagnosed non-skin malignancy and the second leading cause of cancer-related deaths in men in the United States. The approval and commercial success of Novartis’ Pluvicto™ ([¹⁷⁷Lu]Lu-PSMA-617) has revitalized interest in PSMA-targeted radioligand therapy, spurring a wave of new clinical trials and pipeline candidates. However, a subset of patients demonstrates resistance to PSMA-directed therapy, highlighting the urgent need for novel therapeutic strategies to manage advanced, treatment-refractory prostate cancer. Research suggests neuroendocrine-like cells and their hormone neurotensin drive aggressive, androgen-independent prostate cancer via NTSR1—a promising target for radiotheranostics due to its high expression in advanced tumors but absence in healthy tissue.
References:
https://www.mdpi.com/1424-8247/15/10/1292?type=check_update&version=1
https://fusionpharma.com/wp-content/uploads/2023/04/AACR-2023-NTSR1-poster-V4.pdf
Pluvicto™: Mechanism and Expanded Indication
Pluvicto™ (lutetium Lu 177 vipivotide tetraxetan), originally known as [¹⁷⁷Lu]Lu-PSMA-617, is a radioligand therapy that selectively delivers beta radiation to PSMA-positive metastatic prostate cancer cells. Approved by the FDA in March 2022, its indication was broadened in March 2025 to include adults with PSMA-positive mCRPC who have received prior androgen receptor pathway inhibitor (ARPI) therapy and are eligible for delayed taxane chemotherapy.
Figure 1: Chemical structure of [177Lu]Lu-PSMA-617 (Pluvicto™)
ChemAIRS-Designed Synthesis of PSMA-617
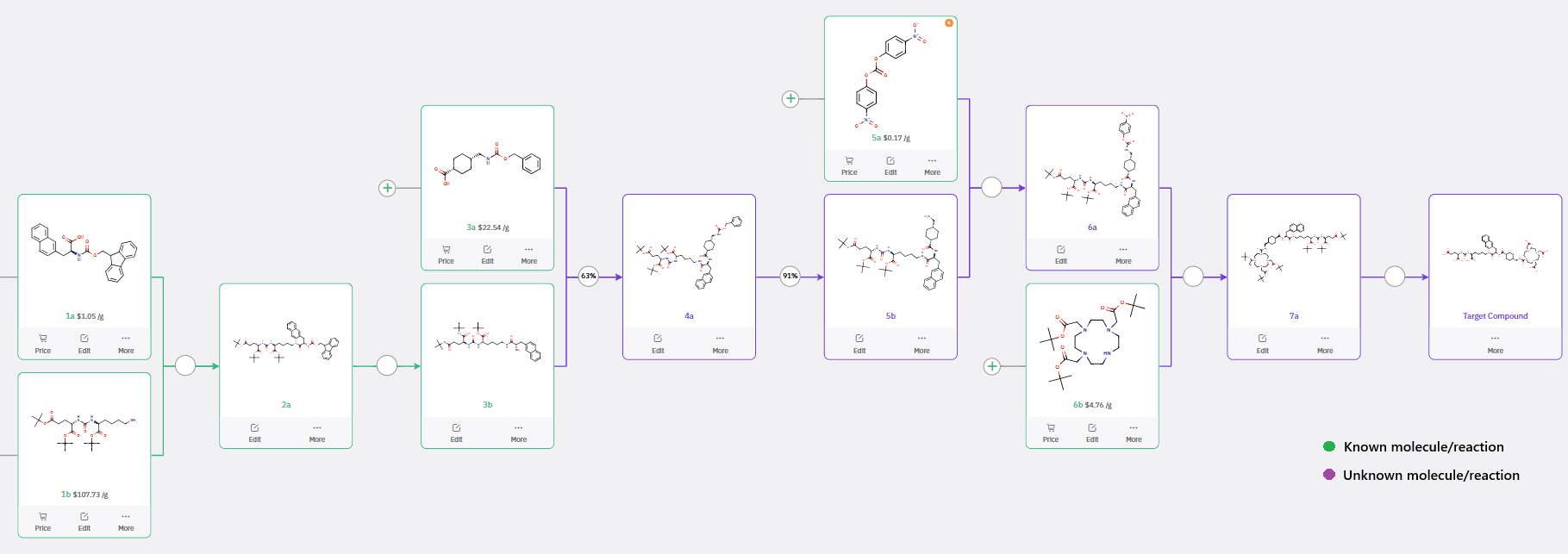
The active pharmaceutical ingredient in Pluvicto™ is the PSMA-targeting ligand, historically designated [¹⁷⁷Lu]Lu-PSMA-617. Vipivotide tetraxetan (PSMA-617) is a modular construct comprising three key elements: a Glu-urea-Lys pharmacophore for high-affinity PSMA binding, a DOTA chelator capable of coordinating either 68Ga or 177Lu, and a linker bridging these domains. Due to its critical role and high cost as a radiopharmaceutical precursor, there is significant demand for efficient synthesis of PSMA-617. To address this, ChemAIRS was tasked with designing an optimized synthetic route utilizing readily available starting materials.
Scheme 1: ChemAIRS-Enabled Synthesis of PSMA-617
Scheme 1 outlines the synthetic route to PSMA-617, initiated by the preparation of intermediate 3b through a two-step sequence starting from the commercially available ADC linker 1b, following a literature-reported procedure. Alternatively, ChemAIRS also provided a viable synthetic pathway for accessing ADC linker 1b de novo (Scheme 2). The route proceeds via amide bond formation between 3a and 3b to furnish 4a, which undergoes catalytic hydrogenolysis to remove the Cbz protecting group, yielding intermediate 5b. Subsequent carbamate formation with 4-nitrophenol affords the activated intermediate 6a, which is then subjected to DOTA conjugation to furnish 7a. Final deprotection under acidic conditions (TFA) delivers the target compound, PSMA-617.
Scheme 2: ChemAIRS provided a synthetic pathway for accessing commercial ADC linker
Advancing NTSR1-Targeted Radiotherapeutics
Neurotensin receptor 1 (NTSR1) is a compelling target for precision oncology due to its high expression in various solid tumors, including colorectal, pancreatic, gastric, neuroendocrine prostate cancers, head and neck squamous cell carcinoma, and Ewing sarcoma. To exploit this, Fusion Pharmaceuticals acquired the beta-emitting radiotherapeutic [¹⁷⁷Lu]-IPN-1087 from Ipsen in April 2021 and enhanced it into the alpha-emitting [²²⁵Ac]-FPI-2059. This next-generation targeted alpha therapy (TAT) harnesses the potent linear energy transfer of actinium-225 for improved tumor cell kill with reduced off-target toxicity. FPI-2059 is now in a Phase I trial, advancing NTSR1-targeted radiopharmaceuticals for challenging solid tumors.
Figure 2: Chemical structure of [225Ac]-FPI-2059
ChemAIRS-Designed Synthesis of NTSR-1–targeting ligand
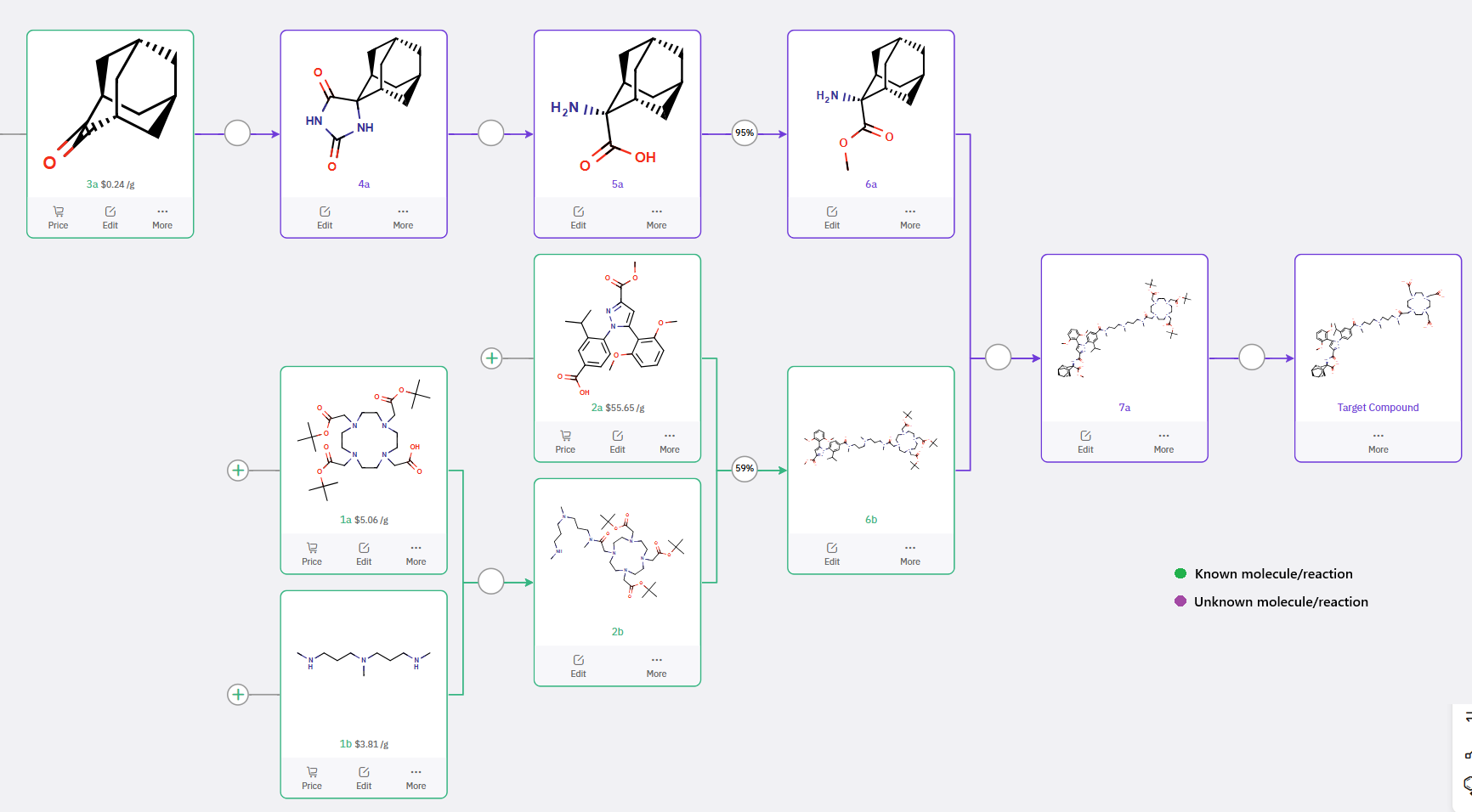
ChemAIRS proposed a synthetic route to the NTSR-1–targeting ligand, comprising four key fragments: 1a, 1b, 2a, and 6a (Scheme 3). The building blocks 1a, 1b, and 2a are commercially available and were utilized to assemble intermediate 6b via an amide coupling reaction, consistent with the procedure described in WO2014086499A1.
Scheme 3: ChemAIRS-Enabled Synthesis of NTSR-1–targeting ligand
The aminoadamantane-2-carboxylate fragment 6a can be prepared in three steps starting from commercially available 2-adamantanone (3a). The initial transformation employs a Bucherer–Bergs strategy to generate the spirohydantoin 4a, which is subsequently hydrolyzed to furnish the adamantyl amino acid 5a and further elaborated to intermediate 6a. Coupling of 6a with 6b via amidation yields 7a, which, upon final deprotection, provides the desired NTSR-1–targeting ligand.
Concluding remarks
ChemAIRS plays a pivotal role in accelerating this innovation by delivering efficient, cost-effective, and scalable synthetic routes for complex radioligand precursors. By integrating domain knowledge with AI-driven retrosynthetic planning, ChemAIRS empowers researchers and manufacturers to keep pace with the rapidly evolving radiopharmaceutical landscape—ultimately improving patient outcomes in advanced prostate and other solid tumors.

![Chemical structure of [177Lu]Lu-PSMA-617 (Pluvicto™)](https://images.squarespace-cdn.com/content/v1/6791175e69de217943348124/e838fc8a-7259-4e9a-bc44-5cd65c830e5a/chemical-structure-of-%5B177lu%5Dlu-psma-617-pluvicto)

![Chemical structure of [225Ac]-FPI-2059](https://images.squarespace-cdn.com/content/v1/6791175e69de217943348124/fd770109-7fdc-4f43-93d5-11292988b646/chemical-structure-of-%5B225ac%5D-fpi-2059)