ChemAIRS-Driven Route Design for Macrocyclic 3CLpro Inhibitors: Streamlining Access to Promising Anti-Coronavirus Therapeutics_EP22
Macrocyclic Inhibitors of 3CLpro: A Promising Strategy Against Coronavirus Replication
The 3C-like protease (3CLpro or Mpro), also known as the main protease, is a key enzyme in coronaviruses, including SARS-CoV-2—the virus responsible for COVID-19. This type of protease is common in many positive-sense RNA viruses and belongs to the cysteine protease family, sharing a chymotrypsin-like fold and using either a catalytic dyad or triad. The Enzyme Commission classifies this group as the main proteinase of SARS coronaviruses. Inhibiting 3CLpro is a promising strategy to stop viral replication, but so far, very few effective inhibitors are available. This highlights the need to develop new compounds that can target this protease or interfere with coronavirus replication.
A recently published patent by Vir Biotechnology describes the design and synthesis of novel macrocyclic compounds that target the 3C-like protease. These macrocycles act as modulators of the protease’s activity, effectively inhibiting coronavirus replication by disrupting a key step in the viral life cycle.
Reference:
WO2025058977 MODULATORS OF CORONAVIRUS 3C-LIKE PROTEASE AND USES THEREOF
Overcoming Synthetic Challenges in 3CLpro Inhibitor Development with ChemAIRS
ChemAIRS proposed innovative synthetic pathways for two patented compounds (44 and 17 in WO2025058977), offering valuable insights that may support chemists in addressing complex synthetic challenges.
ChemAIRS-Enabled Scalable Synthesis of Macrocyclic Compound 44
Scheme 1 outlines the convergent synthesis of macrocyclic intermediate 18b, beginning with the amide coupling of two key fragments, 16a and 16b, to furnish linear precursor 17a. Instead of the conventional macrolactamization strategy reported in WO2025058977, ChemAIRS suggested an alternative approach: a Pd-catalyzed intramolecular Buchwald–Hartwig C–N cross-coupling to forge the macrocyclic framework—an innovative disconnection for enhancing regioselectivity and functional group compatibility in late-stage macrocyclization.
Scheme 1: ChemAIRS-Enabled Scalable Synthesis of Macrocyclic Compound 44 in WO2025058977.
The retrosynthetic analysis of building blocks 16a and 16b are detailed below.
Intermediate 16a – Reductive Cyclization Route
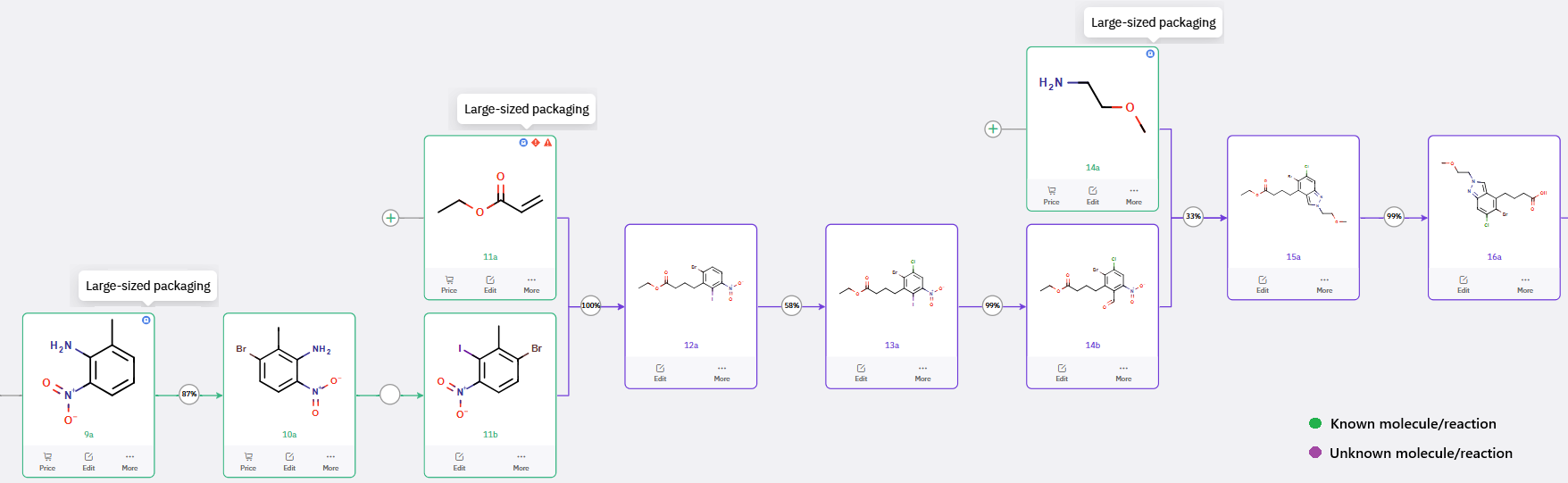
To accommodate large-scale synthesis, ChemAIRS was tasked with identifying robust and scalable routes beginning from readily available commercial feedstocks. Rather than sourcing an indazole derivative directly (as done in the discovery route), the platform identified a cost-effective and operationally simple reductive cyclization strategy: condensation of nitrobenzaldehyde 14b with primary amine 14a, followed by in situ nitro reduction and intramolecular N-arylation to construct the indazole core, yielding intermediate 15a. This route minimizes the need for protected intermediates and streamlines the process for scale-up.
Scheme 2: ChemAIRS-Enabled Scalable Synthesis of Intermediate 16a using Reductive Cyclization strategy.
Intermediate 16b – Arylation Strategy
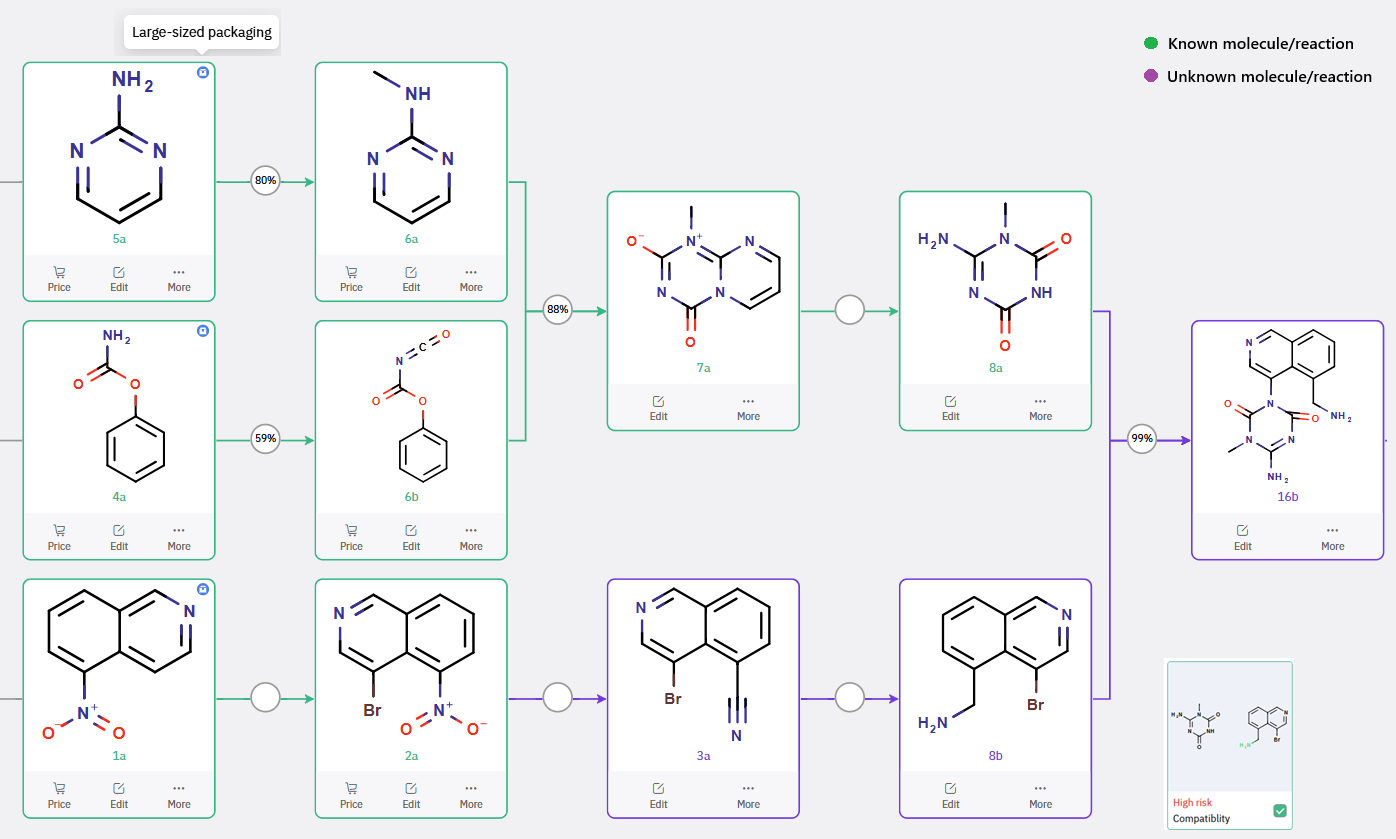
The synthesis of 16b, a dioxo-triazine derivative, also begins from commercially accessible precursors. Key bond construction involves a nucleophilic aromatic substitution or base-promoted arylation between intermediate 8a and aryl bromide 8b. Given the presence of a free amine (–NH₂) moiety—a functionality often sensitive to transition-metal catalysis—ChemAIRS proposed utilizing mild basic conditions (e.g., KOH) to drive the coupling, thus circumventing the need for Cu(I) or Pd(0) catalysts, which may introduce purification or reproducibility challenges at scale.
Scheme 3: ChemAIRS-Enabled Scalable Synthesis of Intermediate 16b via based-promoted arylation.
Streamlined Synthesis of Macrocyclic 17 Using ChemAIRS Retrosynthesis
ChemAIRS proposed a modular assembly of macrocyclic compound 17 through a convergent fragment coupling strategy (Scheme 4). The initial nucleophilic substitution between fragments 19a and 19b delivers intermediate 20b, followed by deprotection to generate activated pentafluorophenyl ester 21a. A thermally promoted macrolactamization in the presence of DIPEA in acetonitrile under reflux conditions affords the desired macrocyclic scaffold 17.
Scheme 4: ChemAIRS-Enabled Streamlined Synthesis of Macrocyclic Compound 17 in WO2025058977.
Interested in exploring detailed synthetic protocols or alternate disconnections for related macrocycles? We’d be happy to assist—please reach out to us at: Contact Chemical.AI
Streamlining Macrocyclic Drug Synthesis with ChemAIRS’ Retrosynthesis Platform
Macrocyclic inhibitors targeting the 3C-like protease offer promising potential against coronaviruses like SARS-CoV-2, but their complex synthesis remains a challenge. This case study highlights ChemAIRS as an AI-driven retrosynthesis platform that identifies scalable routes—demonstrated for two macrocyclic compounds from Vir Biotechnology’s patent. By combining domain expertise and cheminformatics, ChemAIRS accelerates route scouting and overcomes synthetic hurdles, enhancing efficiency and innovation in drug discovery.