ChemAIRS in Action: Accelerating the Discovery and Optimization of Synthetic Routes for ALKS 2680, a Selective Orexin-2 Receptor Agonist developed by Alkermes_EP21
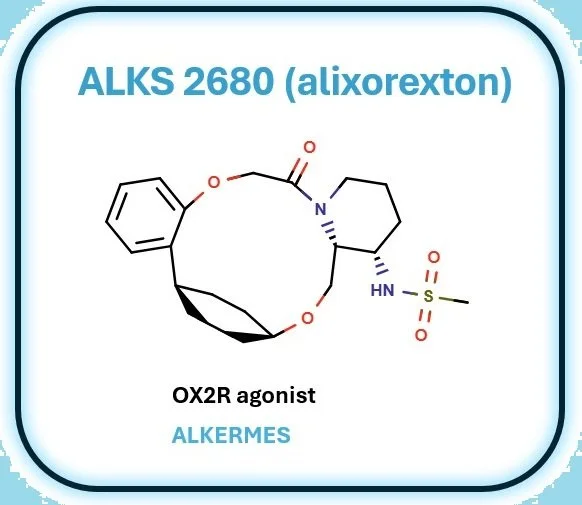
ALKS 2680: A Macrocyclic OX2R Agonist Poised to Revolutionize Narcolepsy Treatment
ALKS 2680 (alixorexton) is a clinical-stage, orally bioavailable macrocyclic agonist of the orexin 2 receptor (OX2R), being advanced by Alkermes for the treatment of narcolepsy and other hypersomnia syndromes. Drawing inspiration from the C-shaped topology of endogenous peptide ligands like orexin-A, the macrocyclic scaffold was strategically designed to impose conformational rigidity, thereby enhancing receptor binding affinity while minimizing the entropic cost of ligand engagement.
Spearheaded by Brian Raymer and the Alkermes discovery team, medicinal chemistry efforts focused on fine-tuning the macrocycle through systematic variation of linker chemistries to optimize OX2R selectivity, CNS penetration, and oral pharmacokinetics—key challenges in the development of small-molecule orexin agonists. Now progressing through Phase 2 trials, alixorexton has the potential to be the first oral agent capable of functionally restoring orexin signaling, a mechanism gaining renewed attention following the 2023 FDA approval of Takeda’s OX2R agonist, mavorelamab (TAK-994), which has reignited interest in this previously elusive drug target.
Reference:
AI-Guided Retrosynthetic Strategies Toward ALKS 2680: A Selective OX2R Agonist
Utilizing the AI-powered retrosynthetic platform ChemAIRS, multiple synthetic routes to the selective OX2R agonist ALKS 2680 were computationally generated and evaluated. The following sections summarize three distinct synthetic strategies:
The published route disclosed by Alkermes
A de novo discovery route enabled by ChemAIRS
A scalable synthetic sequence also proposed by ChemAIRS with an emphasis on practicality and supply chain readiness
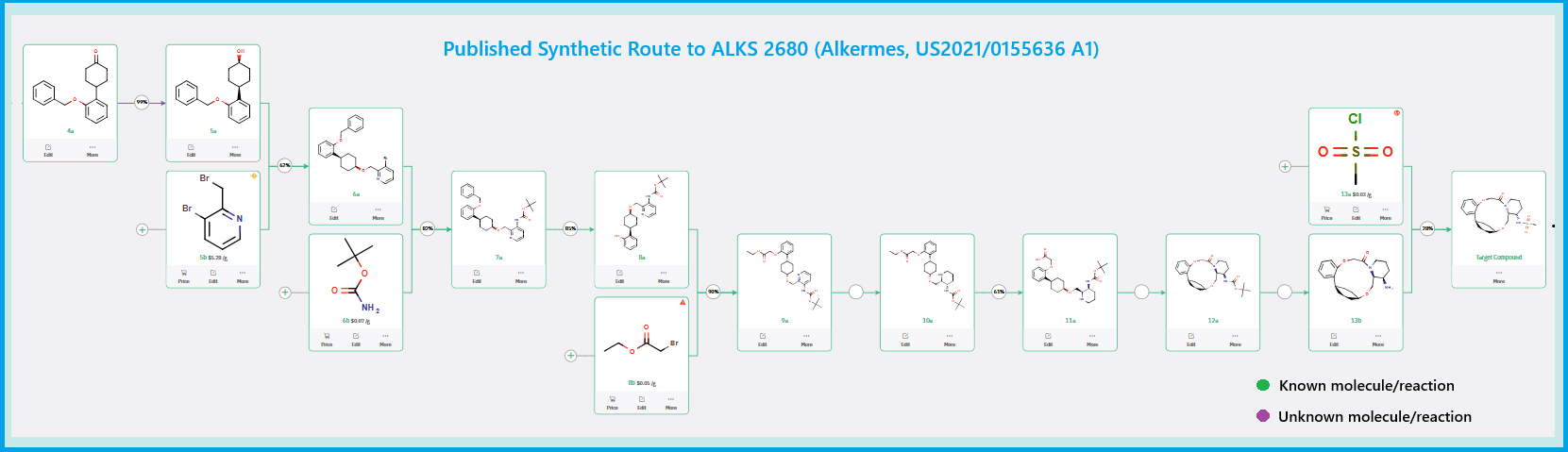
Published Synthetic Route to ALKS 2680 (Alkermes)
The total synthesis of ALKS 2680 was originally reported in US2021/0155636 A1, beginning with a stereoselective reduction of ketone 4a to afford alcohol 5a (Scheme 1). The patented route employed L-selectride for facial selectivity.
To optimize this step, ChemAIRS proposed an alternative asymmetric hydrogenation utilizing an Ir/f-AmphBINOL catalyst system. This enantioselective methodology has demonstrated excellent control over both enantio- and diastereoselectivity, delivering chiral alcohols in up to 99% ee and a cis/trans ratio of 99:1. [DOI: 10.1021/acs.orglett.3c03550]
Another key transformation in the Alkermes route is the macrocyclization of intermediate 11a using HATU/DIPEA in a mixed MeCN/DMF solvent system to deliver macrocycle 12a.
Scheme 1: Alkermes's published synthetic route to ALKS 2680.
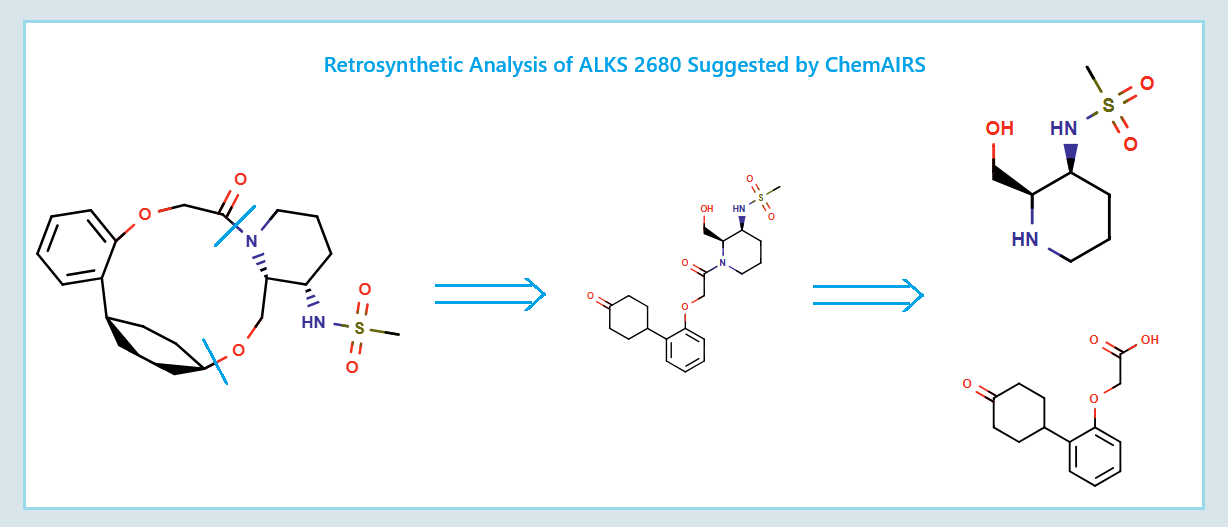
Alternative Discovery Route to ALKS 2680 Proposed by ChemAIRS
Figure 1: ChemAIRS suggested Retrosynthesis Analysis of ALKS 2680.
ChemAIRS generated a novel synthetic sequence with two strategic divergences from the original route (Scheme 2):
Chirality induction was shifted from the phenylcyclohexane core to the piperidine moiety 8a, offering a more modular and potentially convergent approach.
Macrocyclization was deferred to the final stage, thereby reducing the risk of low yields due to ring strain or intermediate instability earlier in the sequence.
Scheme 2: ChemAIRS generated alternative Discovery Route to ALKS 2680.
The conversion of (pyridin-3-yl)methanesulfonamide 7a to (piperidin-3-yl)methanesulfonamide 8a was facilitated by Rh/C-catalyzed hydrogenation in EtOH/AcOH, followed by kinetic resolution using (+)-mandelic acid for stereochemical enrichment (WO2017/135306).
The macrocycle was constructed via intramolecular reductive etherification using Et₃SiH/TMSOTf in DCM (doi.org/10.1002/ejoc.201300135), providing the final scaffold in a late-stage cyclization strategy conducive to structural diversification.
Interested in exploring the capabilities of our Retrosynthesis module? Learn more here ChemAIRS_Retrosynthesis
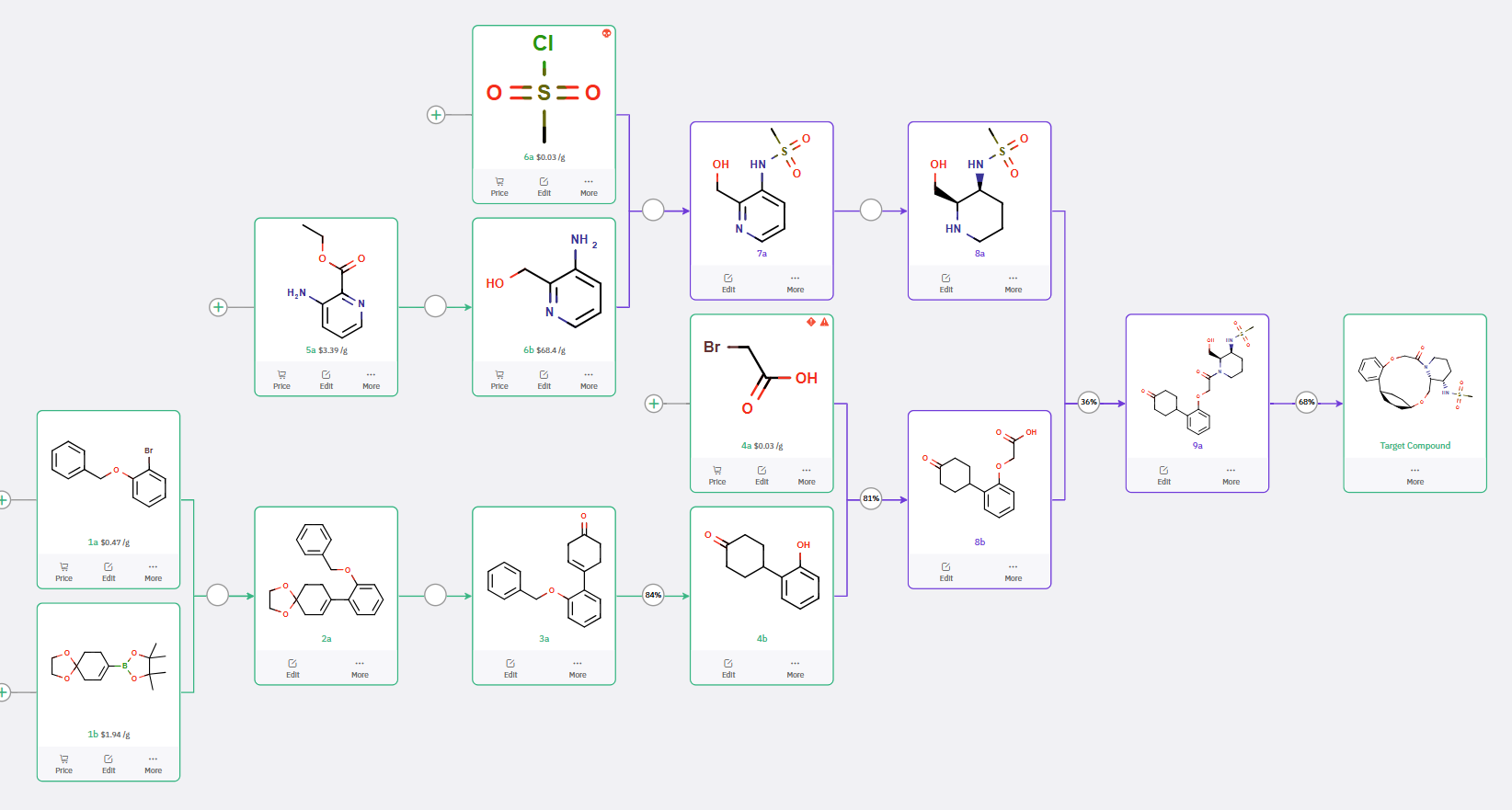
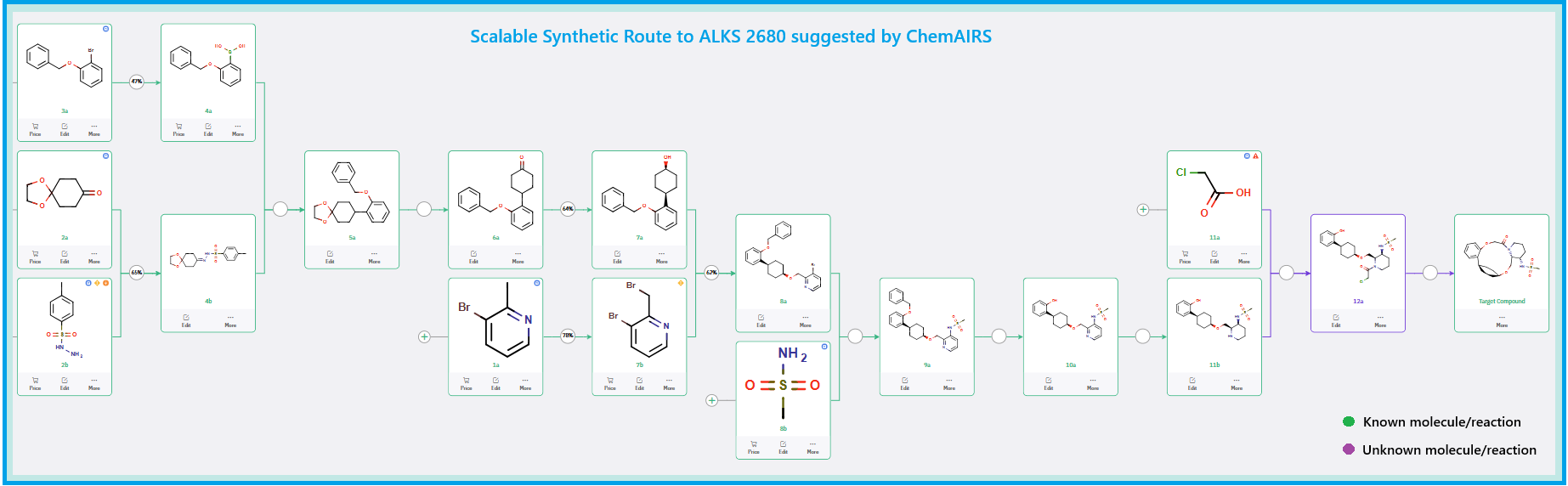
Scalable Synthetic Route from ChemAIRS
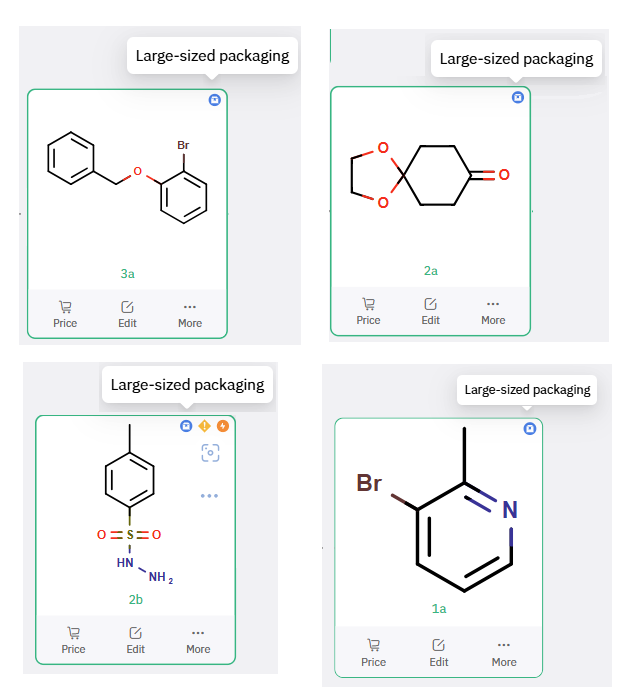
To facilitate process development, ChemAIRS proposed a scale-up route starting from commercially available and cost-effective starting materials, favoring raw materials with established sourcing and regulatory profiles (Scheme 3, Figure 2). The route incorporated robust transformations and minimized reliance on sensitive reagents or cryogenic conditions—key considerations for process chemistry.
Scheme 3: ChemAIRS suggested Scalable Synthetic Route to ALKS 2680.
Figure 2: ChemAIRS Selected Commercially Available Bulk Starting Materials for ALKS 2680 Production.
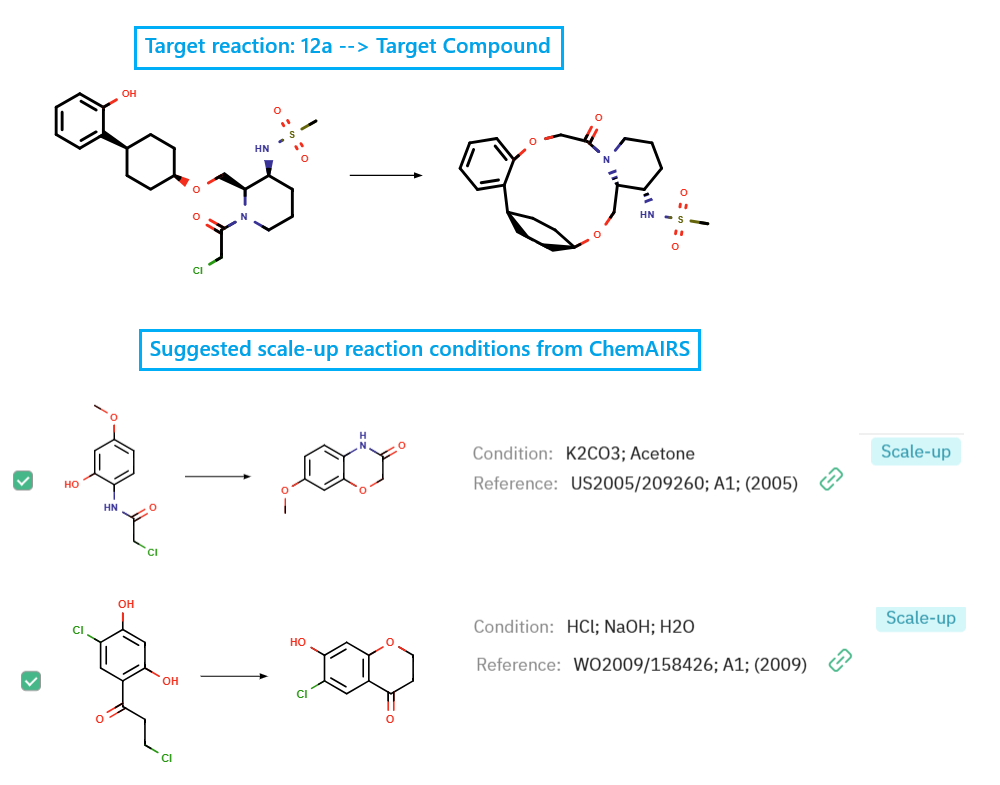
In the final stage, ChemAIRS recommended process-suitable conditions for the key ring-closing step to access the macrocyclic target compound at scale (Figure 3).
Interested in exploring the capabilities of our Process Chemistry module? Learn more here ChemAIRS_Process Chemistry
Figure 3: ChemAIRS suggested scale-up reaction condition for the cyclization step.
From Ideation to Implementation: ChemAIRS as a Catalyst for Medicinal Chemistry Innovation
The deployment of ChemAIRS, an AI-driven retrosynthetic planning platform, enabled the rapid generation and evaluation of diverse synthetic strategies for ALKS 2680, spanning discovery-phase design to process-scale feasibility. By integrating stereochemical precision, strategic late-stage functionalizations, and scalable disconnections, ChemAIRS illustrates the potential of AI tools to augment medicinal chemists’ decision-making and streamline route scouting—from ideation to implementation.