ChemAIRS-Driven Retrosynthesis of Elironrasib (RMC-6291): A Next-Gen KRAS-G12C(ON) Inhibitor_EP20
RMC-6291: A Next-Gen KRAS-G12C(ON) Inhibitor Targeting the Active State
RAS oncogenes—particularly KRAS—are among the most frequently mutated drivers of human cancers, making them critical therapeutic targets. However, therapeutic options for the majority of KRAS-mutant tumors remain limited. While KRAS-G12C is a validated target in non-small cell lung cancer (NSCLC, ~15% of KRAS mutations), current inhibitors focus solely on the inactive, GDP-bound state—leaving the active, GTP-bound form untouched.
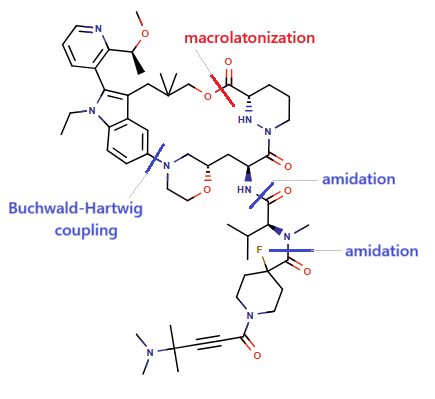
Elironrasib (RMC-6291) represents a next-generation approach by selectively targeting KRAS-G12C(ON), leveraging a sanglifehrin-inspired macrocycle to form a stable tri-complex with KRAS-G12C(ON) and cyclophilin A (CypA). This design confers high selectivity and potency through conformational rigidity and cooperative binding.
Currently in Phase 1 trials (NCT05462717, NCT06128551, NCT06162221), early data from Revolution Medicines show promise in patients resistant to first-gen KRASG12C inhibitors, positioning RMC-6291 as a potential next-generation therapy.
References:
ChemAIRS Retrosynthetic Strategy Toward Elironrasib (RMC-6291)
ChemAIRS proposed a 25-step synthetic sequence toward elironrasib (RMC-6291), demonstrating close alignment with the synthetic strategy disclosed by Revolution Medicines (US 2021/0130303 A1).
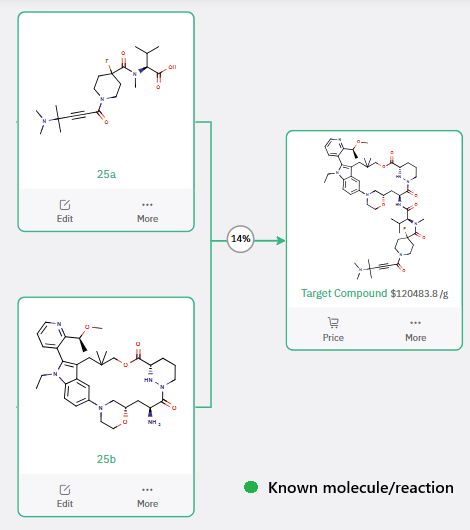
To streamline the retrosynthetic analysis, the macrocyclic target was strategically fragmented into two key intermediates, 25a and 25b (Scheme 1).
Interested in exploring the capabilities of our Retrosynthesis module? Learn more here ChemAIRS_Retrosynthesis
Scheme 1: Key fragmentation intermediates for Elironrasib retrosynthesis
ChemAIRS-Generated Synthetic Pathway for fluoropiperidine Intermediate 25a
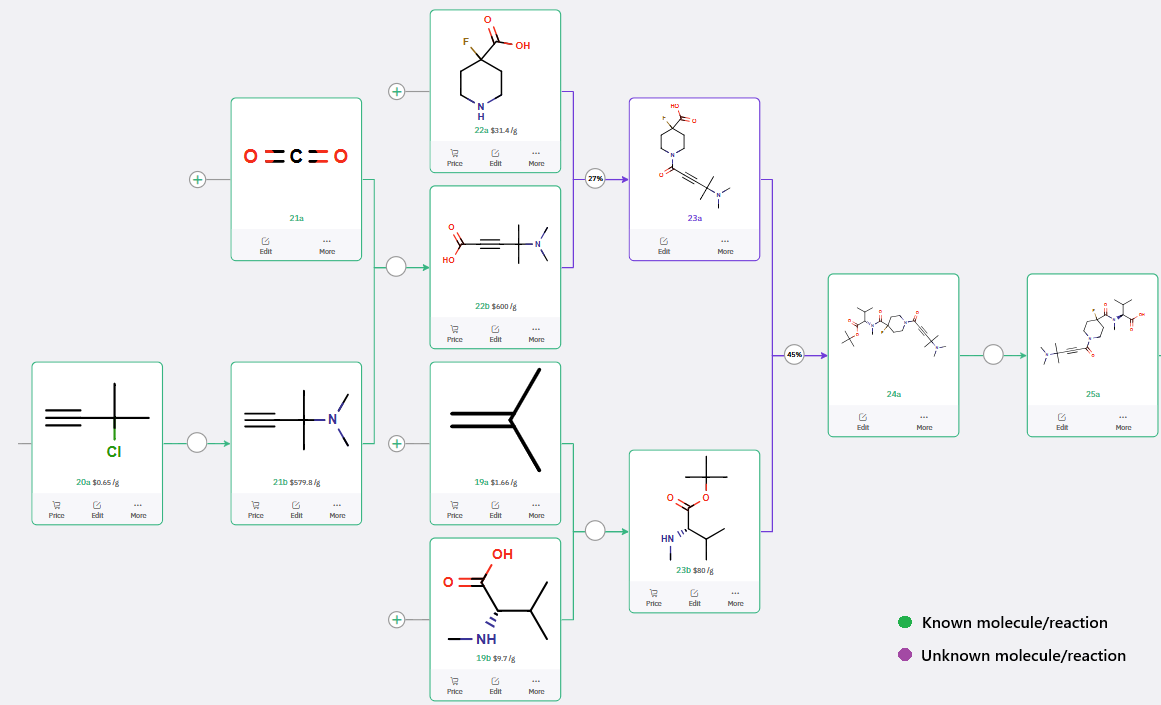
ChemAIRS designed a 6-step synthetic route to intermediate 25a (Scheme 2), representing a more concise alternative to the 8-step route reported in the patent literature. The key disconnection involves an amide bond formation between fragments 23a and 23b, furnishing intermediate 24a. Subsequent deprotection affords the desired intermediate 25a.
Additionally, ChemAIRS identified several commercially available building blocks (e.g., 22b, 23b), facilitating procurement and synthesis planning.
Scheme 2: ChemAIRS-Generated Synthetic Pathway for fluoropiperidine Intermediate 25a
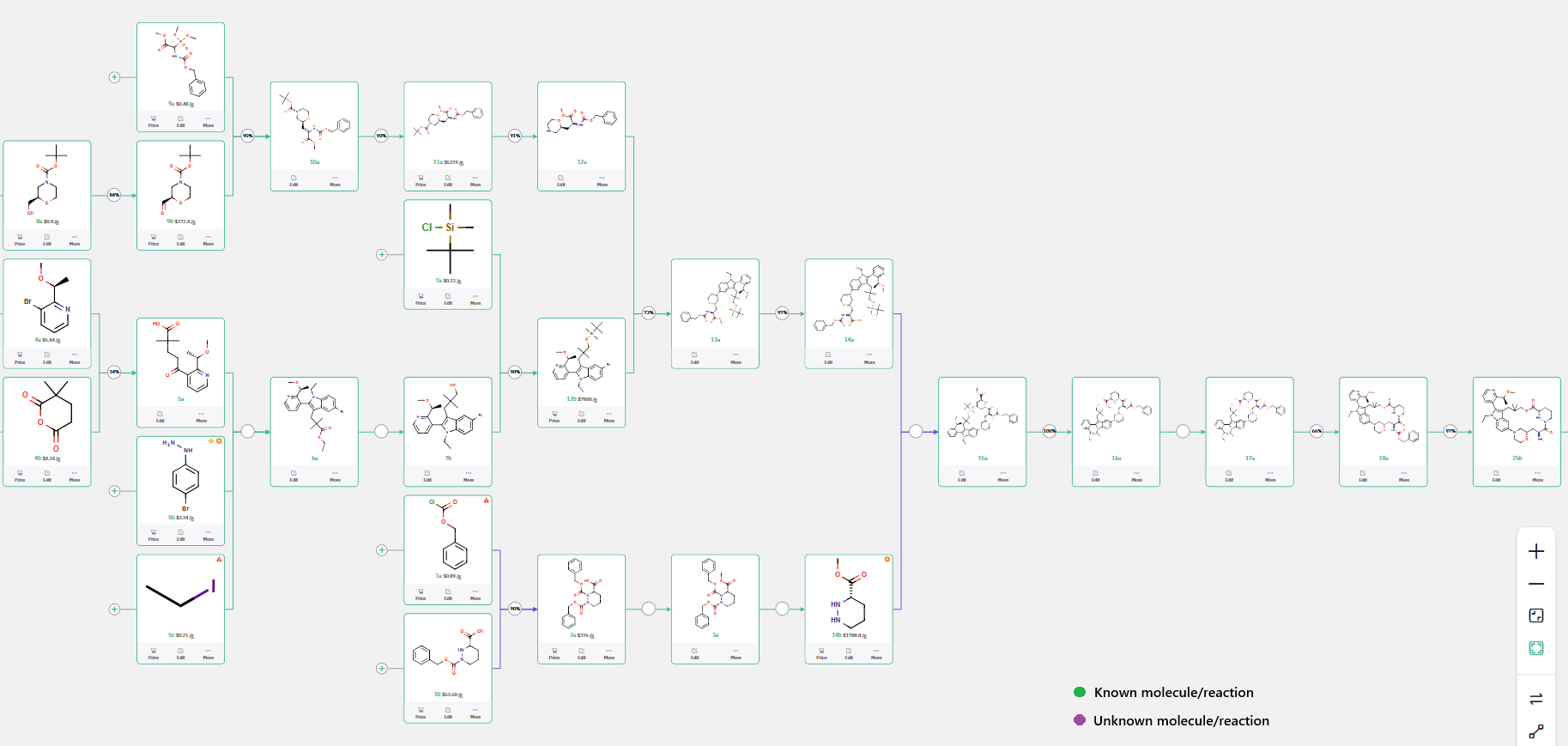
Synthetic Route to Macrocyclic Intermediate 25b: Cost Reduction and Side Reaction Mitigation
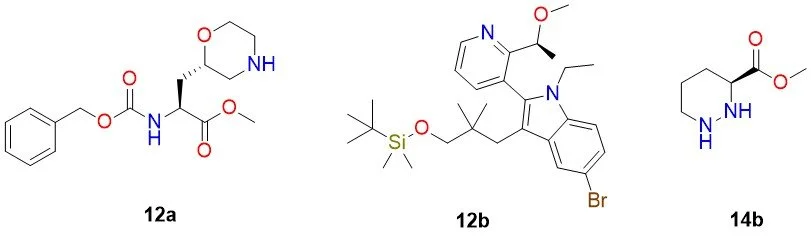
The synthesis of intermediate 25b was approached by fragmenting the macrocycle into three building blocks: 12a, 12b, and 14b (Figure 1).
Figure 1: Retrosynthetic fragmentation of intermediate 25b into building blocks 12a, 12b, and 14b.
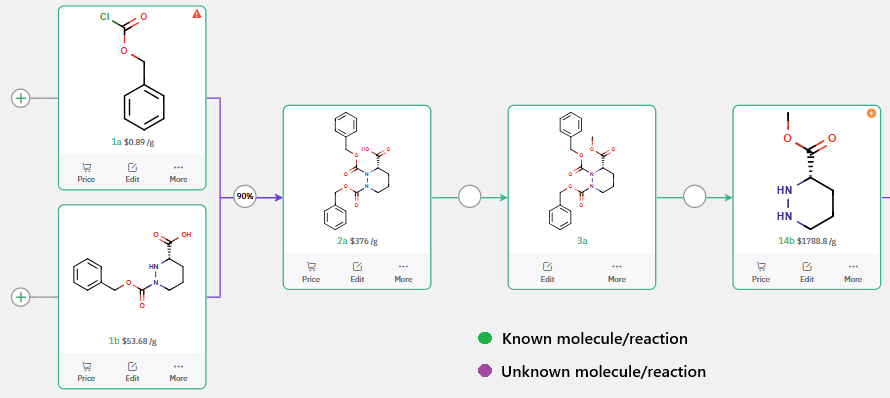
While the preparation of 12a and 12b follows reported procedures (US2021/130303), ChemAIRS proposed a de novo 3-step synthesis of 14b (methyl (S)-hexahydropyridazine-3-carboxylate), circumventing the need for costly commercial procurement (Schemes 3 and 4).
Scheme 3: ChemAIRS-Generated Synthetic Pathway for Macrocyclic Intermediate 25b
Scheme 4: Cost-effective route to (S)-Methyl hexahydropyridazine-3-carboxylate (14b): ChemAIRS-enabled 3-step synthesis avoids procurement challenges
The convergent step involves amide coupling between intermediates 14a and 14b to yield 15a. Although this transformation is disclosed in literature, potential side reactions are not discussed. ChemAIRS addressed this gap by identifying plausible side products associated with this coupling (Figure 2).
Further steps include macrolactonization to generate the macrocyclic intermediate 18a, followed by CBz deprotection to deliver 25b.
Figure 2: ChemAIRS identified potential competing side reactions
Closing the Synthesis Loop: From Fragmentation to Successful Route Reconstruction
The ChemAIRS platform successfully reconstructed a 25-step synthetic route to Elironrasib (RMC-6291), closely mirroring the strategy reported by Revolution Medicines. By breaking down the macrocyclic target into two key fragments (25a and 25b), ChemAIRS enabled a modular and efficient retrosynthetic approach. In addition to pathway design, ChemAIRS identified commercially available starting materials and highlighted potential side reactions, providing valuable insights for synthetic planning and risk mitigation. These results underscore ChemAIRS's potential as a practical tool for accelerating complex molecule synthesis in drug discovery workflows.