Blog
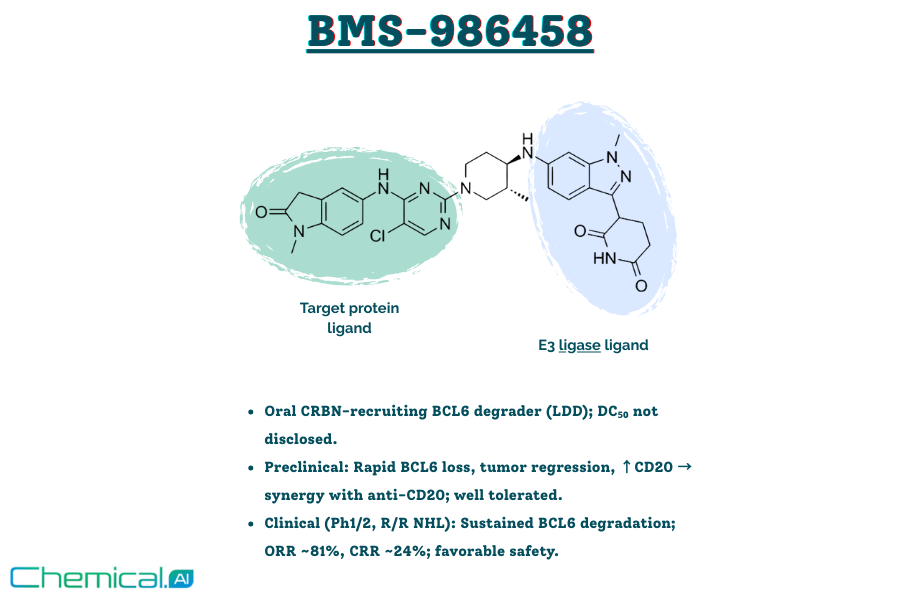
Bridging Biology and Chemistry: BCL6, BMS-986458, and AI-Predicted Routes to Scalable Degraders
This blog explores the role of BCL6 as a central lymphoma driver and the clinical progress of Bristol Myers Squibb’s oral degrader BMS-986458. It also discusses AI-driven retrosynthesis tools like ChemAIRS, which propose palladium-free, scalable synthetic routes to overcome key challenges in PROTAC development.

Expanding the Therapeutic Toolbox: New Modalities for Modern Drug Hunters
This article reframes drug discovery around a central strategic question: not simply ‘Can we inhibit this target?’ but ‘What’s the optimal way to modulate this biology for the right patient?’ Through a case study on dabrafenib’s paradoxical MAPK activation, it shows why conventional inhibition can fail and how next-generation BRAF inhibitors and targeted protein degraders avoid those pitfalls.
It then zooms out to map the full range of modern modalities, from antibodies and RNA therapeutics to ADCs, PROTACs, molecular glues, and tri-complex inhibitors, and the frameworks that guide their selection based on biology, mechanism, and practical constraints. With examples like Revolution Medicines’ macrocyclic molecular-glue approach to RAS, the piece positions modality choice as a competitive advantage in creating the next generation of precision medicines.

ChemAIRS-Driven Retrosynthesis of Elironrasib (RMC-6291): A Next-Gen KRAS-G12C(ON) Inhibitor_EP20
The ChemAIRS platform successfully reconstructed a 25-step synthetic route to Revolution Medicines' groundbreaking KRAS inhibitor, elironrasib (RMC-6291). This next-generation therapeutic leverages a sanglifehrin-inspired macrocycle to form a stable tri-complex with KRAS-G12C(ON) and cyclophilin A (CypA), achieving exceptional selectivity through conformational rigidity.
Key ChemAIRS contributions:
Modular retrosynthesis: Deconstructed the macrocycle into two manageable fragments
Supply chain optimization: Identified commercially available starting materials
Risk mitigation: Flagged potential side reactions for synthetic planning
Route validation: Closely mirrored Revolution Medicines' published strategy
By combining macrocyclic drug design with AI-driven synthesis planning, ChemAIRS demonstrates how computational tools can accelerate the development of complex targeted therapies.

Enhancing the Retrosynthetic Strategy for Orforglipron (LY3502970) Using ChemAIRS: Developing a More Streamlined and Efficient Synthetic Route Suggestion_EP18
ChemAIRS revolutionized the synthesis of Eli Lilly’s oral GLP-1 agonist Orforglipron—optimizing key intermediates and streamlining scale-up conditions for commercial-ready production. Accelerate your metabolic drug development with AI-driven retrosynthesis.
