ChemAIRS Optimizes the Synthesis of Novo Nordisk’s Glucose-Sensitive Derivatives_EP19
Novo Nordisk’s Diboron-Based Glucose-Sensitive Insulins: A New Chapter in Diabetes Therapy
The discovery of insulin over a century ago revolutionized diabetes management, but its use carries the risk of hypoglycemia, deterring patients from aggressive glucose control. To address this, researchers have long pursued glucose-sensitive insulin variants that enable better glycemic control with fewer injections.
Novo Nordisk is advancing this approach with glucose-sensitive diboron insulin derivatives, incorporating aryl boronic acid or aryl boroxol moieties. These modifications allow insulin activity to be glucose-dependent, reducing hypoglycemia risk while extending half-life, potentially lowering injection frequency. Recent patents (WO2019092125, WO2025016992, WO2024223511, WO2020201041) highlight their progress in developing next-generation insulin therapies for improved diabetes management.
References:
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2019092125
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2025016992&_cid=P10-M835MI-50045-2
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2024223511&_cid=P21-M6AX4C-75941-5
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2020201041
ChemAIRS Outlines Synthesis Strategies for Diboron-Based Glucose-Sensitive Derivatives
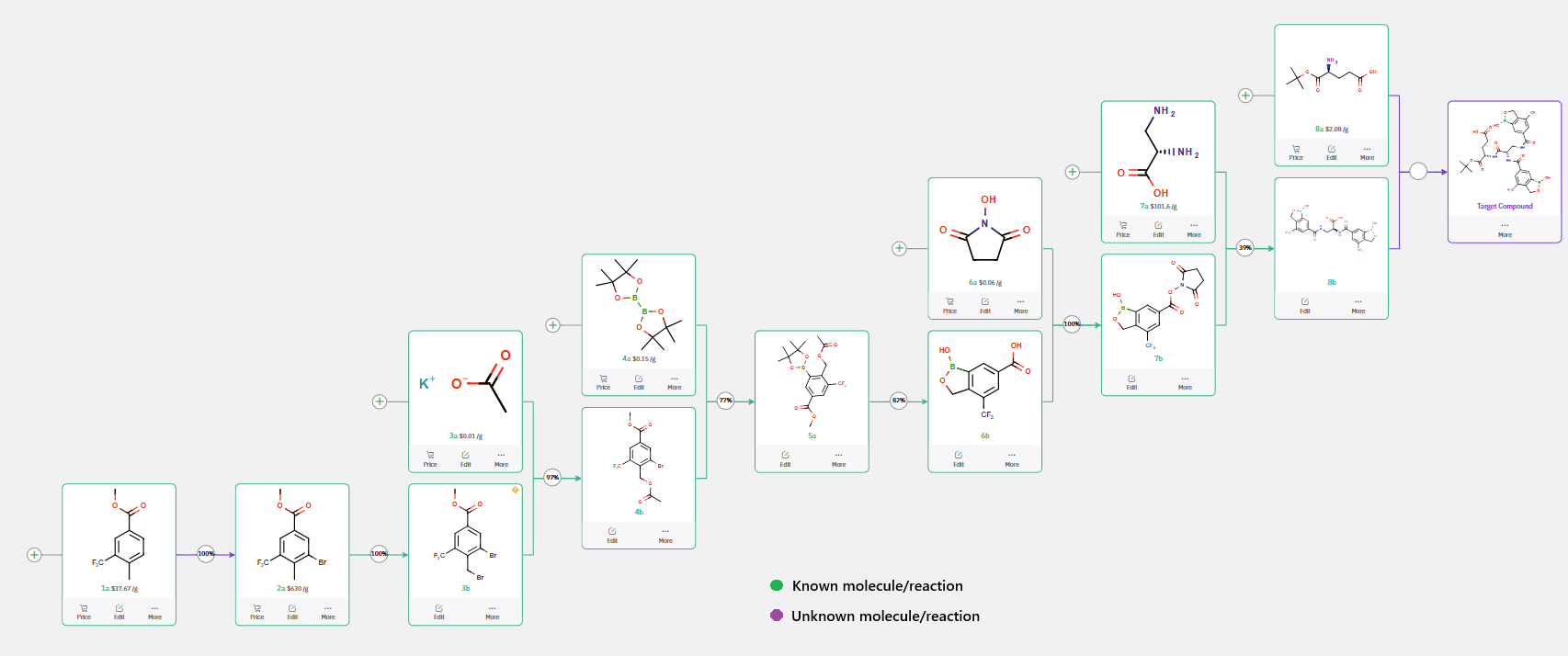
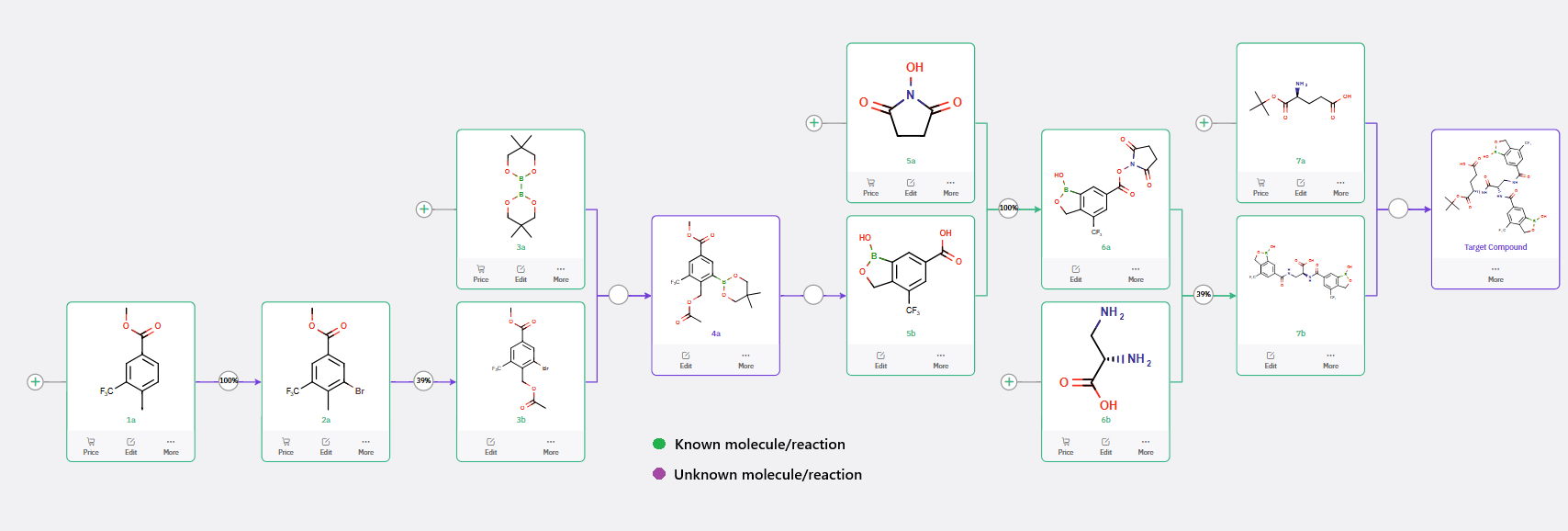
ChemAIRS has proposed multiple synthetic routes for the preparation of a diboron-containing building block, one of which closely parallels the approach reported by Novo Nordisk (WO2019092125, Scheme 1).
Scheme 1: ChemAIRS Outlines Synthesis Strategies for Diboron-Based Glucose-Sensitive Derivatives
The ChemAIRS-designed synthetic route commences from the commercially available methyl 4-methyl-3-(trifluoromethyl)benzoate (1a, Scheme 1) and proceeds through a seven-step sequence to furnish the key (oxaborole-carboxamido)propanoic acid intermediate (8b). In the final step, rather than employing a Glu-functionalized 2-chlorotrityl chloride resin, ChemAIRS proposes a more streamlined approach, directly coupling 8b with L-glutamic acid tert-butyl ester (H-Glu-OtBu) in a single step using DIPEA/HATU/DMF-mediated peptide bond formation.
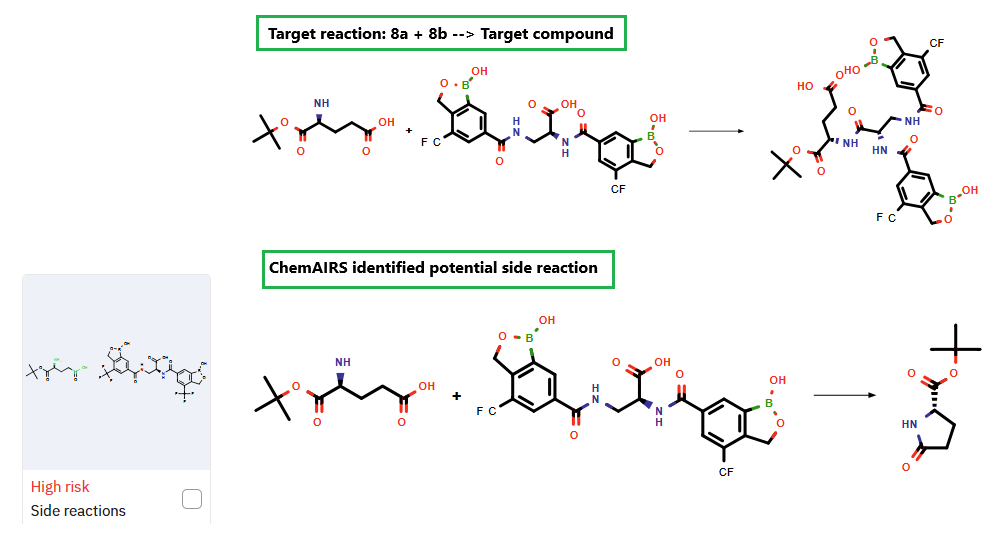
Additionally, the platform identified a potential competing side reaction in the final transformation, as illustrated in Figure 1.
Figure 1: ChemAIRS identified a potential competing side reaction
ChemAIRS Enables Streamlined Synthesis of Dioxaborolanes via Microwave-Ir Catalyzed Borylation
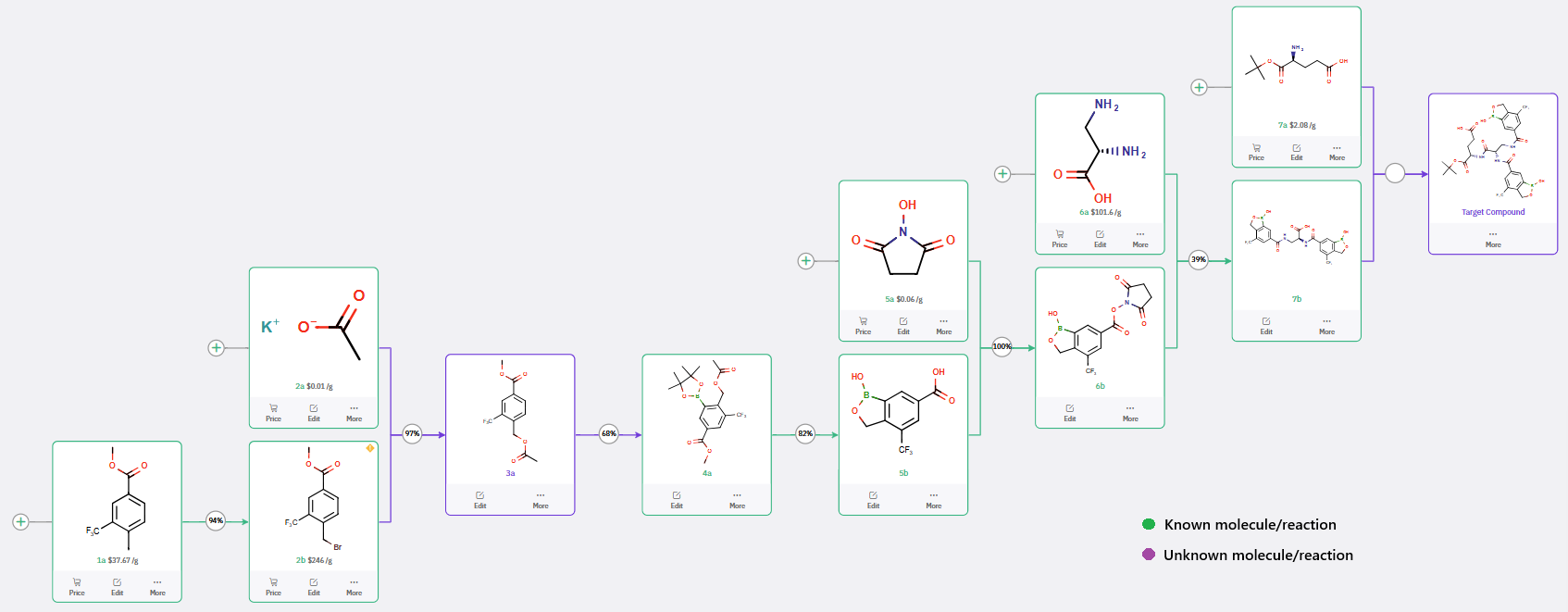
Beyond proposing a synthetic pathway closely aligned with the literature precedent, ChemAIRS has also identified strategies to enhance the efficiency of the sequence. As depicted in Scheme 2, the introduction of the dioxaborolane moiety (compound 4a) can be streamlined from a four-step process to a three-step sequence by leveraging a microwave-assisted Ir-catalyzed borylation utilizing the [Ir(OMe)cod]₂/dtbpy/MTBE system.
This approach offers a highly efficient, regioselective, and environmentally favorable alternative, enabling completion of the borylation reaction within 1 hour—significantly reducing the reaction time compared to the 12-day thermal method described in the original patent (WO2024223511, WO2020201041, and WO2019092125).
Scheme 2: ChemAIRS reveals optimized strategies for enhanced synthesis of Diboron-Based Glucose-Sensitive Derivatives
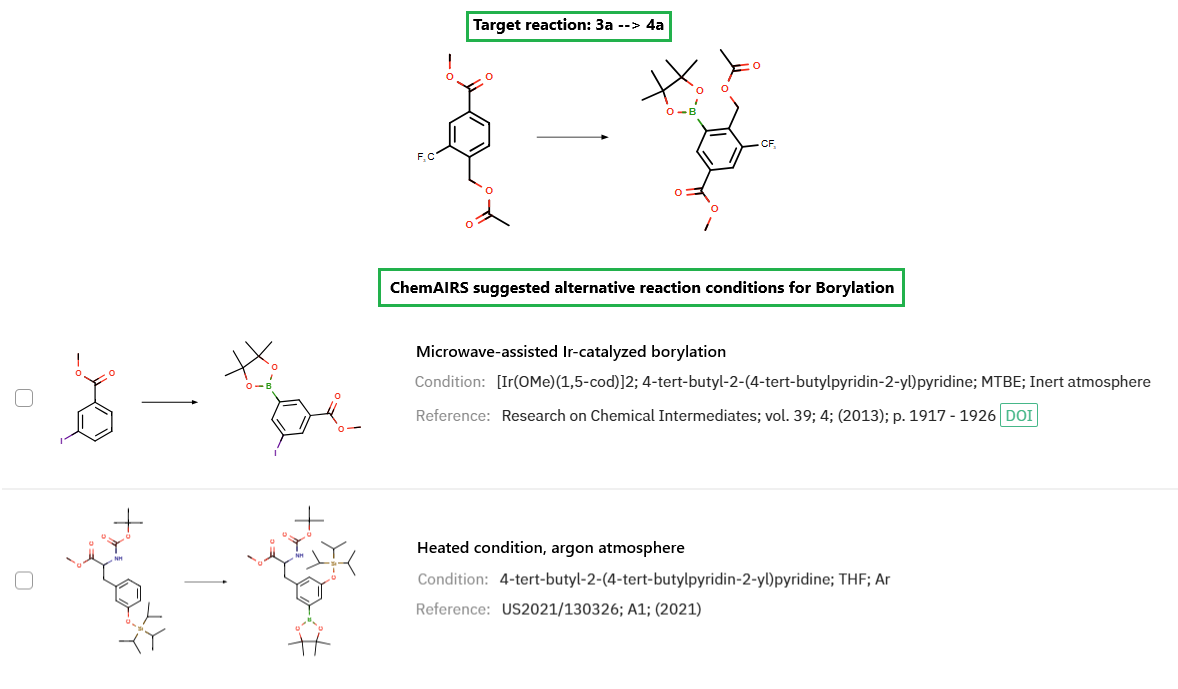
Furthermore, the [Ir(OMe)cod]₂/dtbpy system remains effective under standard thermal conditions in an argon atmosphere, achieving gram-scale conversion within 24 hours without microwave irradiation (Figure 2).
Figure 2: ChemAIRS identified alternative borylation conditions to enhance reaction performance
ChemAIRS-Suggested Alternative Pathway to Novo Nordisk Glucose-sensitive diboron derivatives with Scalable Reaction Conditions
In addition to the synthetic routes described above, ChemAIRS has proposed an alternative method for introducing the dioxaborolane motif via Miyaura borylation using bis(neopentyl glycolato)diboron (B₂(neop)₂) instead of B₂Pin₂ (Scheme 3). Employing a Pd(PCy₃)₂Cl₂/B₂(neop)₂/base catalytic system, this transformation proceeds with an exceptionally low palladium loading (0.04 mol%) (https://pubs.acs.org/doi/10.1021/acs.joc.2c01649).
This strategy demonstrates the feasibility of employing minimal Pd catalyst quantities for scalable organoboron synthesis.
Scheme 3: ChemAIRS optimizes Miyaura borylation: B₂(neop)₂ replaces B₂Pin₂, enabling ultra-low Pd with Pd(PCy₃)₂Cl₂/base
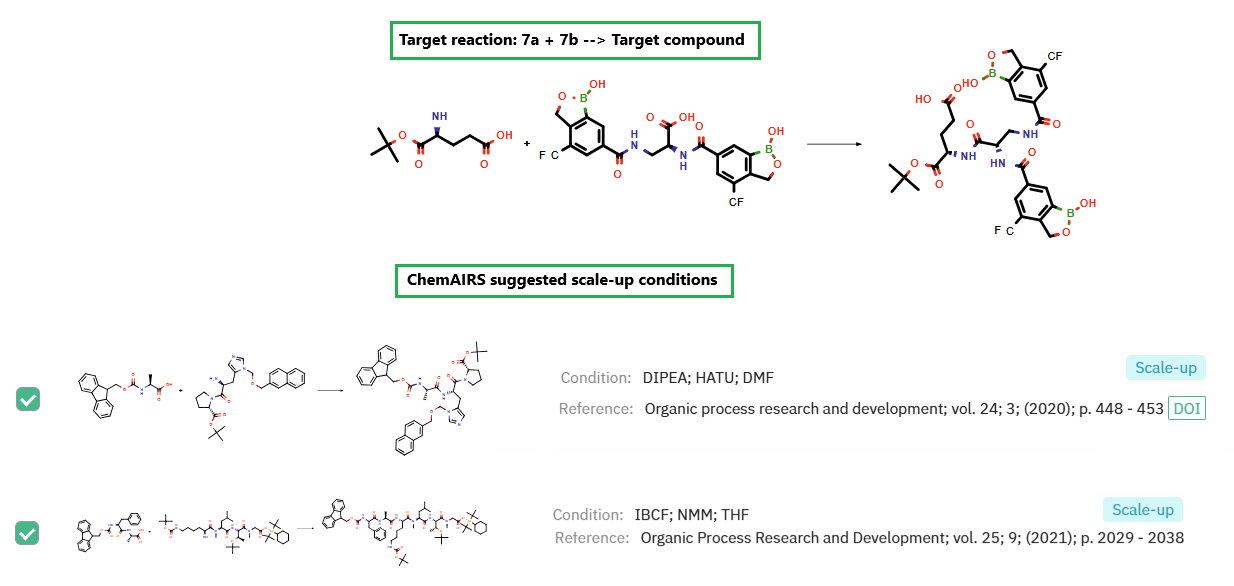
Additionally, ChemAIRS has proposed optimized scale-up conditions for the final step (Figure 3).
Interested in exploring the capabilities of our Process Chemistry module? Learn more here ChemAIRS_Process Chemistry
Figure 3: ChemAIRS delivers optimized reaction parameters for robust and scalable synthesis
From Insight to Implementation
ChemAIRS successfully designed and optimized synthetic routes for a diboron-containing building block, improving efficiency and scalability. Using microwave-assisted Ir-catalyzed borylation and ultra-low Pd Miyaura borylation with B₂(neop)₂, the platform reduced reaction times and costs. It also assessed side reactions for robust scale-up, enhancing synthetic accessibility for glucose-sensitive derivatives. Future integration of predictive analytics with experimental validation will further refine workflows, advancing medicinal and process chemistry.