Leveraging ChemAIRS to Investigate Synthetic Strategies for SGR-1505, a MALT1 inhibitor from Schrodinger_EP09
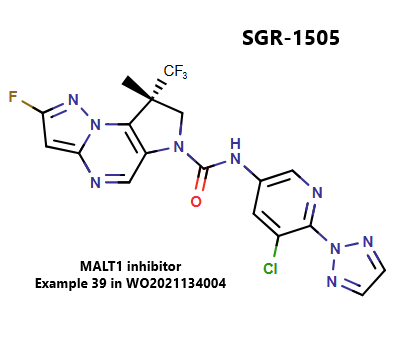
Targeting MALT1 Protease Activity: SGR-1505 as a Novel Therapeutic Approach for Cancer Treatment
MALT1 is an intracellular protein that regulates lymphocyte activation, survival, and proliferation through NF-κB signaling. Its protease activity has emerged as a therapeutic target, particularly in cancers where NF-κB pathways are implicated. MALT1 is associated with various cancers, including hematological malignancies like mantle cell lymphoma and CLL, as well as solid tumors such as lung adenocarcinoma and breast cancer. Research is ongoing to evaluate MALT1 inhibitors for cancer treatment, though none are currently approved.
Schrödinger designed SGR-1505, an allosteric inhibitor of MALT1, which is undergoing Phase 1 trials for advanced B-cell lymphoma.
Reference: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2021134004
Optimization of Synthetic Routes for SGR-1505 Using ChemAIRS
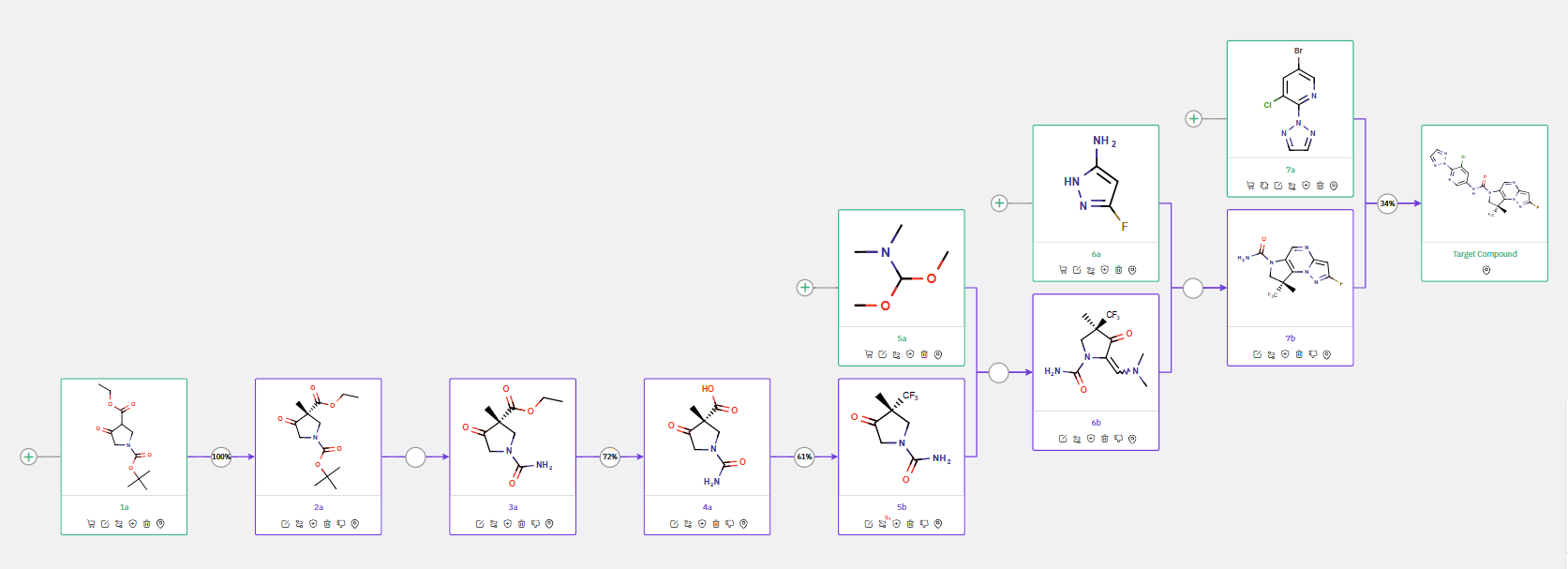
ChemAIRS evaluated the synthetic routes for producing SGR-1505, a promising candidate for B-cell lymphoma treatment. The initial synthetic approach began with a cyclocondensation reaction between intermediates 2a and 2b, followed by hydrolysis of intermediate 3a, and a subsequent Curtius rearrangement to form urea intermediate 5a (Scheme 1).
Scheme 1: ChemAIRS predicted a synthetic pathway for an SGR-1505
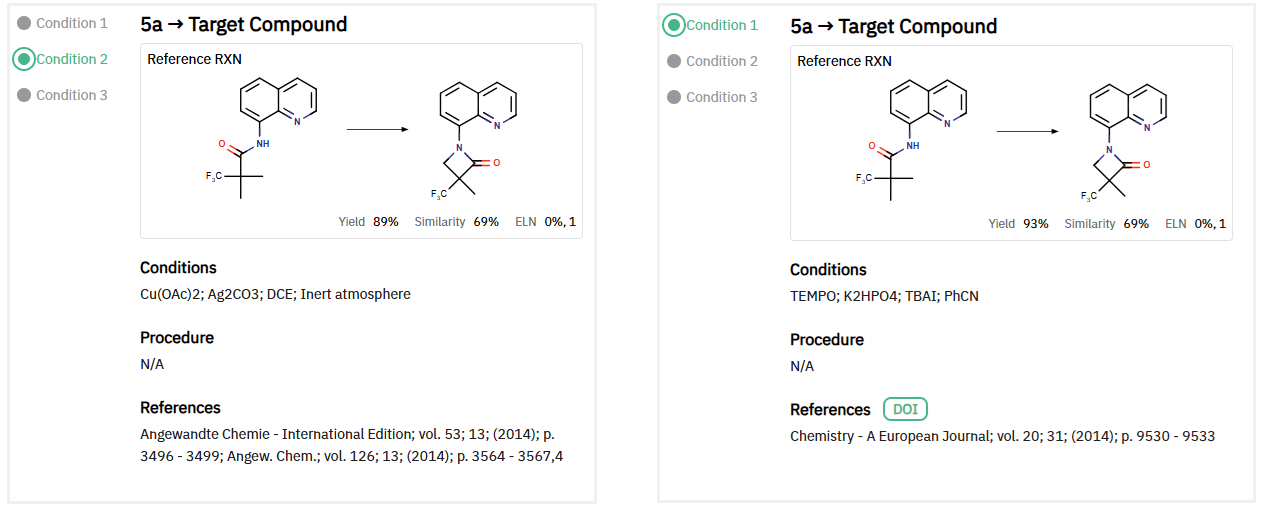
For the final step, ChemAIRS suggested two catalytic pathways: a copper-catalyzed intramolecular oxidative amidation or a nickel-catalyzed intramolecular cyclization (Figure 1). The final target molecule could exist as a racemic mixture, which can be resolved via chiral column chromatography. This synthetic approach, particularly the use of the Curtius rearrangement, presents a new idea to previously published routes for SGR-1505 synthesis.
Figure 1: ChemAIRS suggested two reaction conditions for intramolecular cyclization
Alternative Synthetic Approach to SGR-1505
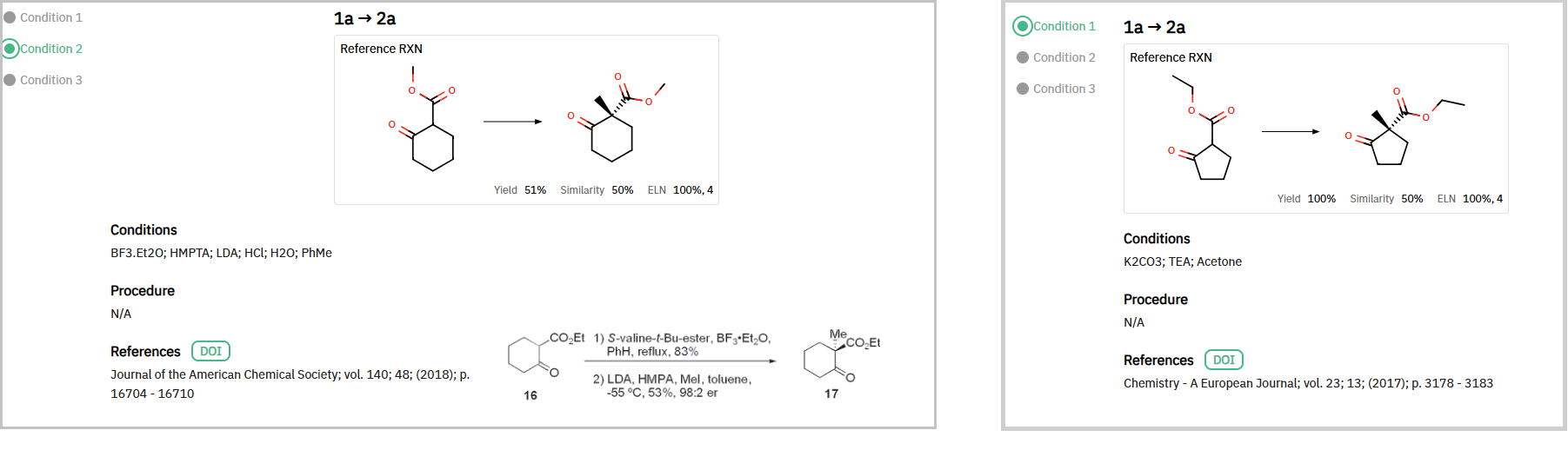
An alternative route to the final API began with an introduction of chirality early in synthesis, yielding intermediate 2a (Scheme 2). ChemAIRS proposed using asymmetric methylation with (S)-valine t-Bu-ester as a chiral auxiliary (Figure 2). This enantioselective reaction eliminates the need for costly chiral purification steps later in the synthesis by generating the desired stereochemistry in situ.
Scheme 2: An alternative synthetic strategy to obtain SGR-1505
Figure 2: Suggested reaction conditions for asymmetric methylation
For the final step, two synthetic strategies were suggested. The first involved a palladium-catalyzed amination between intermediates 7a and 7b, though the high cost of substrate 7a prompted an alternative route. ChemAIRS proposed a transamidation between amine 7a and urea 7b, offering a more cost-effective method for constructing the target compound (Figure 3).
Figure 3: Alternative substrates for 7a in the last step
In conclusion, ChemAIRS not only provided efficient strategies for obtaining the final API but also offered valuable insights to inspire further innovation in synthetic methodologies.
Interested in exploring the capabilities of our Retrosynthesis module? Learn more here: ChemAIRS_Retrosynthesis