Utilizing ChemAIRS to Explore Synthetic Strategies of AB521, an HIF-2⍺ inhibitor from Arcus Bioscience_EP10
Targeting HIF-2α: AB521 (Casdatifan) as a Promising Antitumor Agent in Cancer Therapy
Hypoxia-inducible factors (HIFs) regulate cellular responses to low oxygen levels, with HIF-1α and HIF-2α often overexpressed in cancers. HIF-2α has been linked to poor prognosis in glioblastoma, neuroblastoma, head and neck squamous cell carcinoma, and non-small cell lung cancer. This makes HIF-2α a key target for therapeutic intervention in cancer and related conditions.
Arcus Biosciences Inc. recently unveiled the chemical structure of AB521 (casdatifan), a small molecule inhibitor of the oxygen-sensing transcription factor HIF-2⍺ that has demonstrated potent antitumor activity in xenograft models of renal cell cancer (RCC).
Reference:
WO2021188769 TETRALIN AND TETRAHYDROQUINOLINE COMPOUNDS AS INHIBITORS OF HIF-2ALPHA (wipo.int)
Prediction of Convergent Synthesis for Casdatifan (AB521) using ChemAIRS
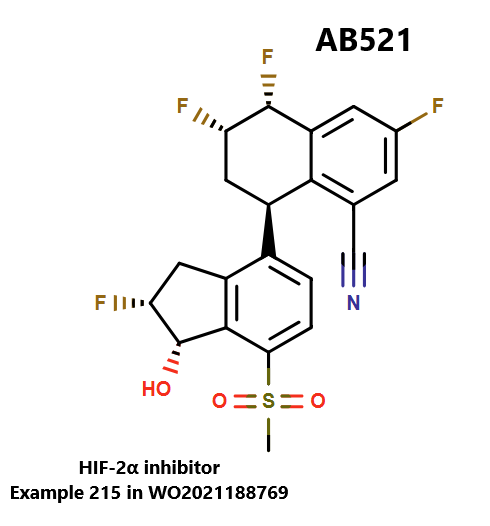
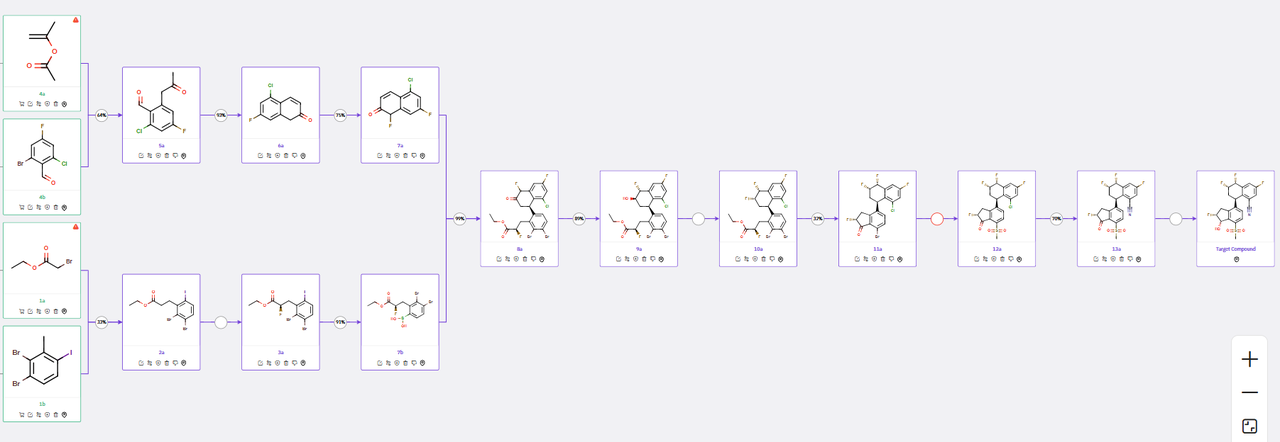
The molecular architecture of Casdatifan (AB521) consists of four densely substituted ring systems containing five stereocenters, presenting considerable complexity in the overall synthetic design. ChemAIRS developed several convergent synthetic routes for AB521, where critical intermediates could be synthesized in parallel to facilitate the construction of the final target molecule.
The synthetic route begins with a key coupling reaction between intermediates 10a and 10b, derived from readily accessible commercial precursors such as dibromo benzaldehyde (6b) and bromo indanone (1a) (Scheme 1). The synthesis of 10a follows a four-step sequence as proposed by ChemAIRS, differing from the multistep approach outlined in the corresponding patent. This route initiates with the coupling of fluoroketone 6a and dibromo benzaldehyde 6b, followed by an intramolecular aldol condensation to yield compound 8a. Subsequent steps involve ketone reduction and Pd-catalyzed cyanation, ultimately affording intermediate 10a.
Scheme 1: ChemAIRS predicted a synthetic pathway for AB521
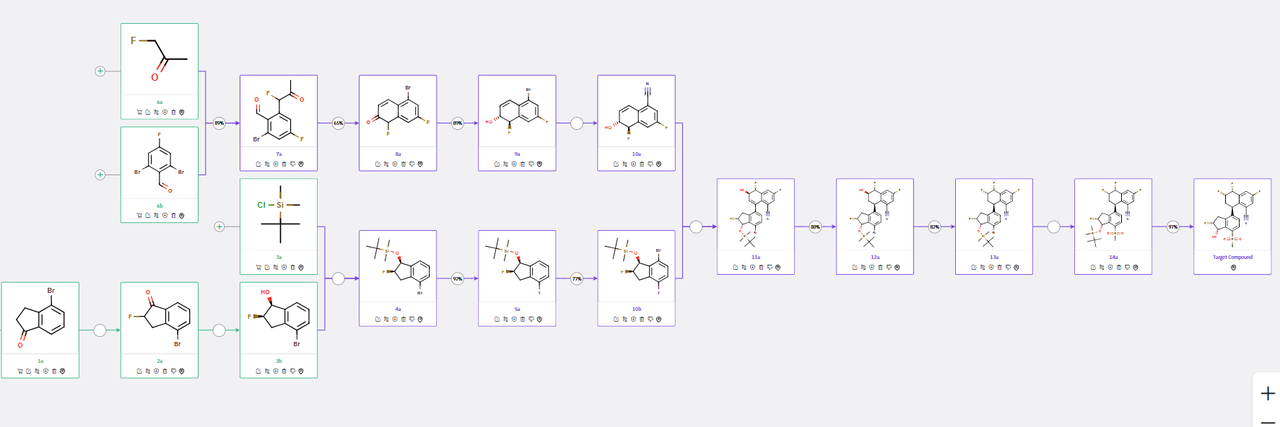
Given the aldehyde functionality in 6b, which poses a potential risk for the coupling reaction, an alternative two-step synthesis of intermediate 7a was developed (Scheme 2).
Scheme 2: An alternative way to synthesize intermediate 7a
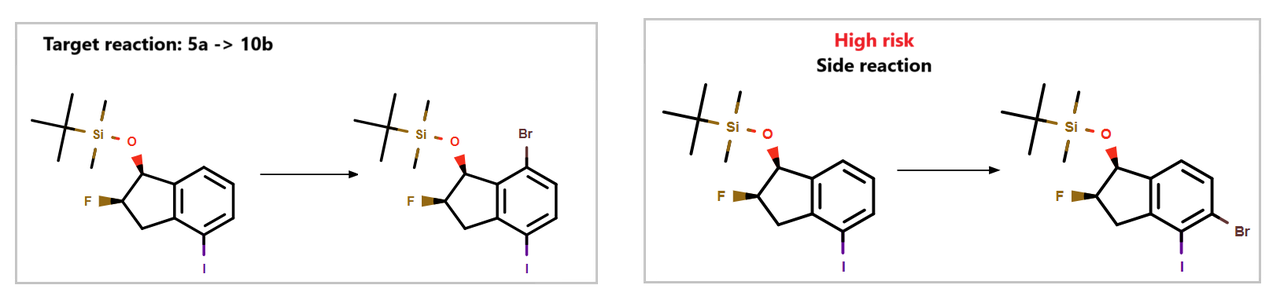
Additionally, our system flagged a risk associated with the synthesis of 10b, as highlighted in Figure 1.
Figure 1: Potential side reactions for the synthesis of intermediate 10b
ChemAIRS Proposed Alternative Synthetic Approach to AB521
ChemAIRS also suggested an alternative pathway to AB521, illustrated in Scheme 3.
Interested in exploring the capabilities of our Retrosynthesis module? Learn more here: ChemAIRS_Retrosynthesis
Scheme 3: Alternative synthetic route for AB521
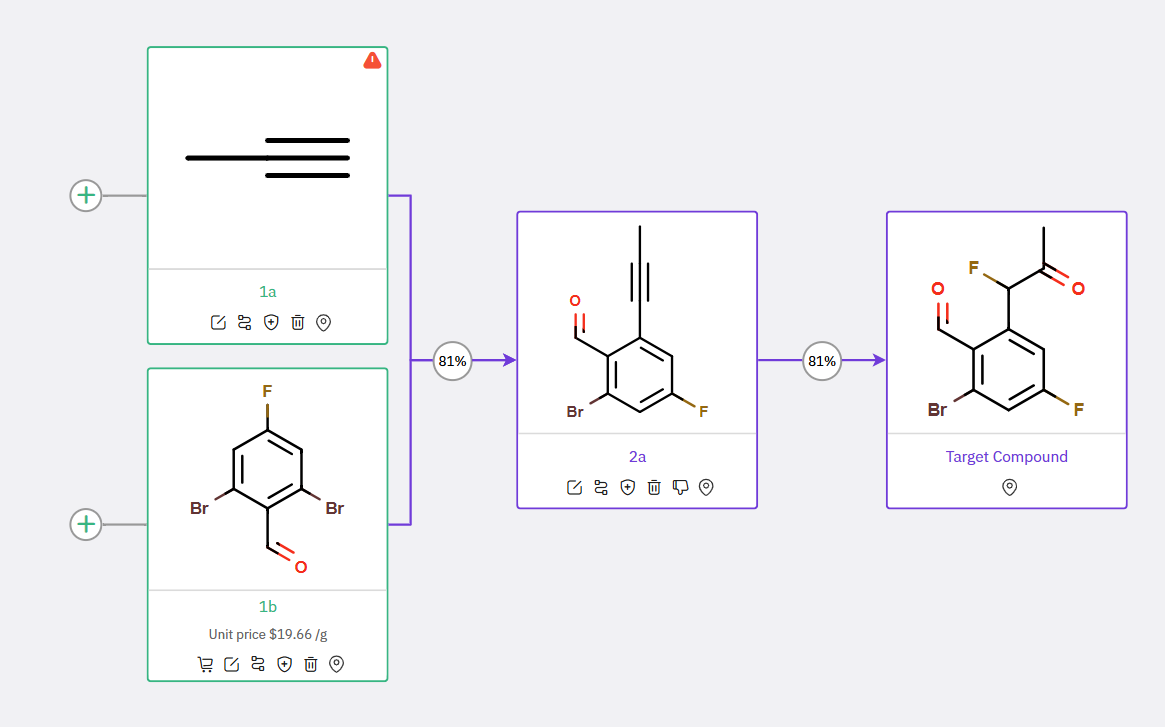
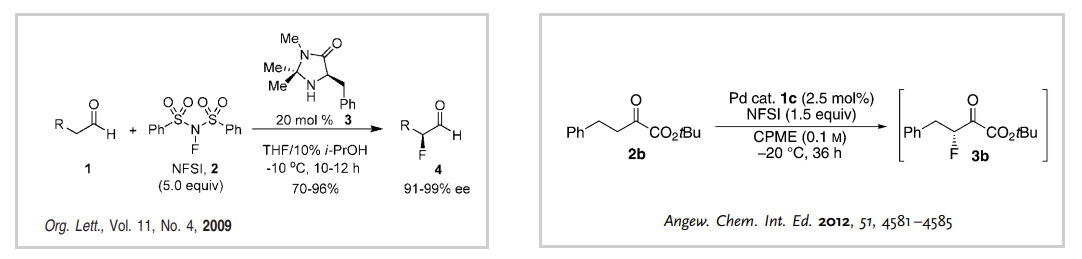
In the first part of the synthesis, intermediate 7a can be obtained as a mixture of isomers post-fluorination, which can be resolved via column chromatography. Importantly, in the latter stages of the synthesis, our system recommended an enantioselective mono-fluorination strategy to prepare compound 3a, as demonstrated in the reference reaction (Figure 2).
Figure 2: Reference reactions for the enantioselective mono-fluorination to intermediate 3a
In conclusion, ChemAIRS has proven its capability to identify alternative synthetic routes for complex drug molecules, providing unique pathways that have the potential to save researchers time and effort, thus enabling a more efficient exploration of synthetic strategies.