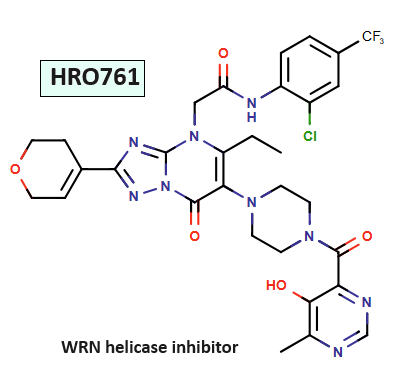
Streamlining the Synthesis of Novartis’ WRN Inhibitor HRO761 with ChemAIRS: A Promising Therapeutic for MSI Cancers_EP05
Targeting WRN in MSI Cancers: A Novel Clinical Approach from Novartis
Genetic screens have identified the Werner syndrome RecQ helicase (WRN) as a synthetic lethal target in microsatellite instability (MSI) cancer cells. Despite advances in treatment with immune checkpoint inhibitors, there is an unmet need for the treatment of MSI cancers.
Novartis researchers have developed a potent and selective WRN inhibitor, HRO761, which is now undergoing its first human clinical trial to assess its pharmacokinetics, safety, tolerability, and preliminary anti-tumor efficacy in MSI colorectal cancer and other MSI solid tumors.
Reference:
https://www.nature.com/articles/s41586-024-07350-y
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2022249060&_cid=P10-LZ1SAF-47576-1
ChemAIRS Predicted the Reported Synthetic Route for the WRN inhibitor HRO761
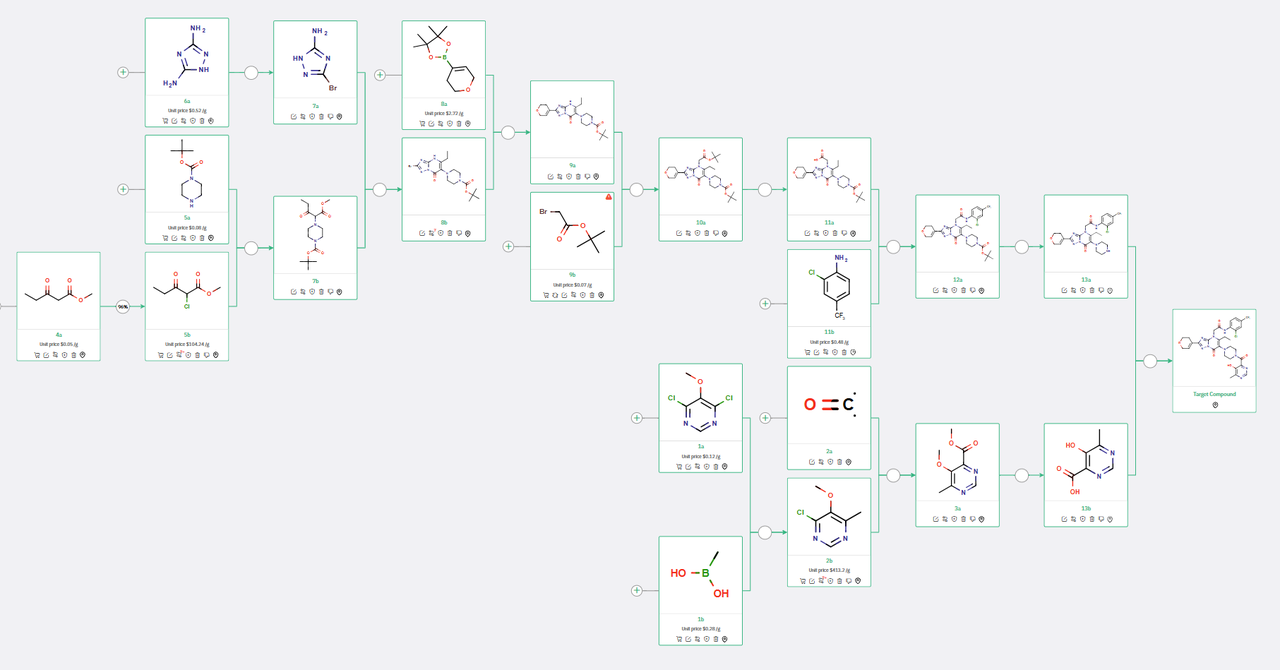
ChemAIRS proposed a feasible 13-step synthetic route (Scheme 1) wherein the final target compound was synthesized through an amide coupling reaction between intermediates 13a and 13b, closely mirroring the methodology reported by Novartis.
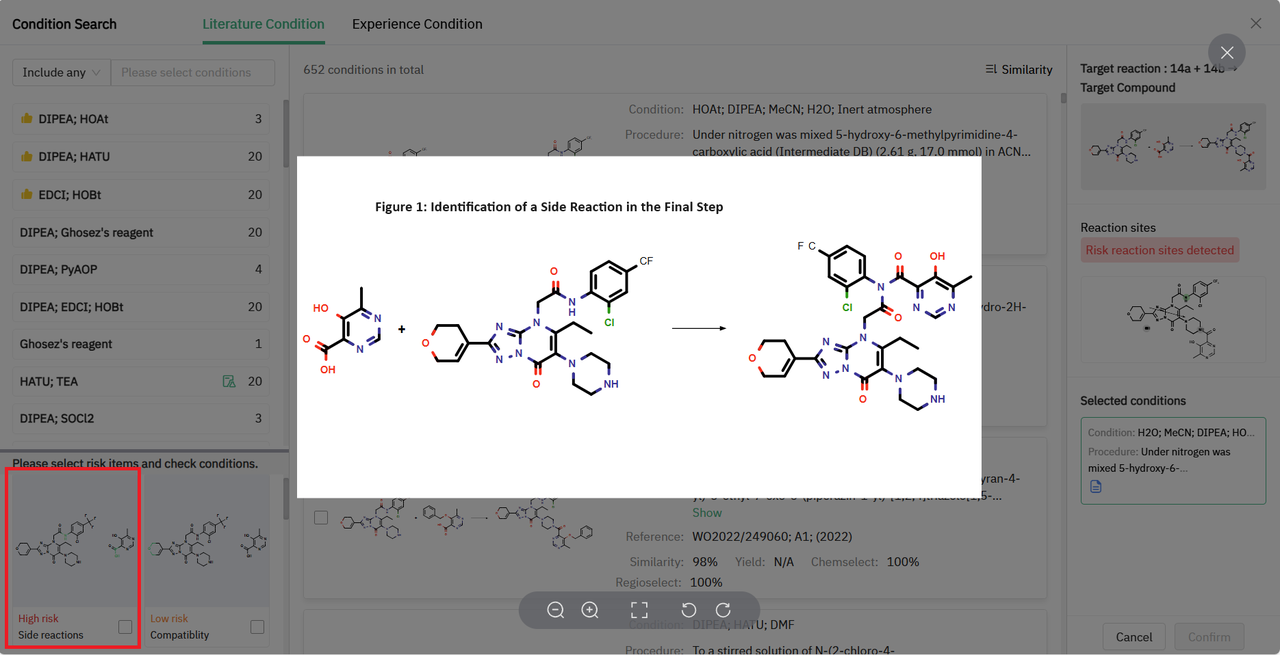
Moreover, ChemAIRS identified a potential risk in the final step, specifically a possible side reaction as depicted in Figure 1. Notably diverging from the existing patent, ChemAIRS suggested a 3-step synthesis for the key intermediate 13b. This procedure commenced with a Suzuki coupling reaction to form the chloropyrimidine derivative 2b, which was subsequently transformed into the corresponding methyl ester 3a via palladium-catalyzed carbonylation.
Scheme 1: ChemAIRS predicted a synthetic pathway for Novartis's WRN inhibitor HRO761, closely aligned with the published route
Figure 1: ChemAIRS identified side reaction risk for the last-step synthesis of target molecule
Revised Synthetic Strategy from ChemAIRS to Minimize Reaction Risks
Acknowledging a risk associated with the final step of Scheme 1, which involved an amide coupling reaction between intermediate 13a and pyrimidine-carboxylic acid 13b, we tasked ChemAIRS to propose an alternative approach as illustrated in Scheme 2.
In this revised scheme, the pyrimidine moiety was introduced earlier in synthesis via an amide coupling reaction between 11a and 11b, followed by a demethylation step to yield 13b. In the final step, trifluoromethylaniline 13a reacted with the intermediate 13b, a strategy intended to minimize the risk of the side reaction mentioned in Figure 1.
Scheme 2: ChemAIRS suggested an alternative synthetic pathway for WRN inhibitor HRO761 to mitigate final step risks
In conclusion, the ChemAIRS Retrosynthesis tool demonstrated its robust capability in identifying potential risks and proposing innovative solutions. Its capability to deviate from existing patents and suggest a synthesis for key intermediates underscores its value in accelerating synthetic organic chemistry projects and ensuring successful outcomes.
Interested in exploring the capabilities of our Retrosynthesis module? Learn more here: ChemAIRS_Retrosynthesis