Leveraging ChemAIRS to Unveil Synthetic Strategies for Selective Cannabinoid Receptor 2 Inverse Agonists_EP11
Advances in CB2 Inverse Agonists: Exploring Therapeutic Potential and Selectivity
In recent years, pharmacology has made significant strides in understanding various receptors in the human body, especially those associated with the endocannabinoid system. A notable area of interest involves CB2 inverse agonists, a class of compounds that bind to and modulate the CB2 receptor (CB2R), one of the primary cannabinoid receptors, which is pivotal in regulating immune responses and inflammation. Despite their potential therapeutic benefits, especially in models of arthritis and neuroinflammation, CB2R antagonists and inverse agonists remain largely underexplored.
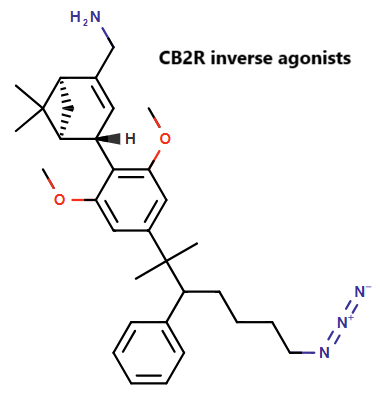
A collaborative effort between ETH, Oregon Health & Science University, and Roche has resulted in the discovery of novel CB2R inverse agonists. These compounds exhibit high binding affinity for CB2R and demonstrate excellent selectivity against CB1R, minimizing unwanted central nervous system effects.
Reference: https://pubs.acs.org/doi/10.1021/acscentsci.3c01461?ref=PDF
Synthetic Strategies for selective CB2R inverse agonists: ChemAIRS-Assisted Route Development
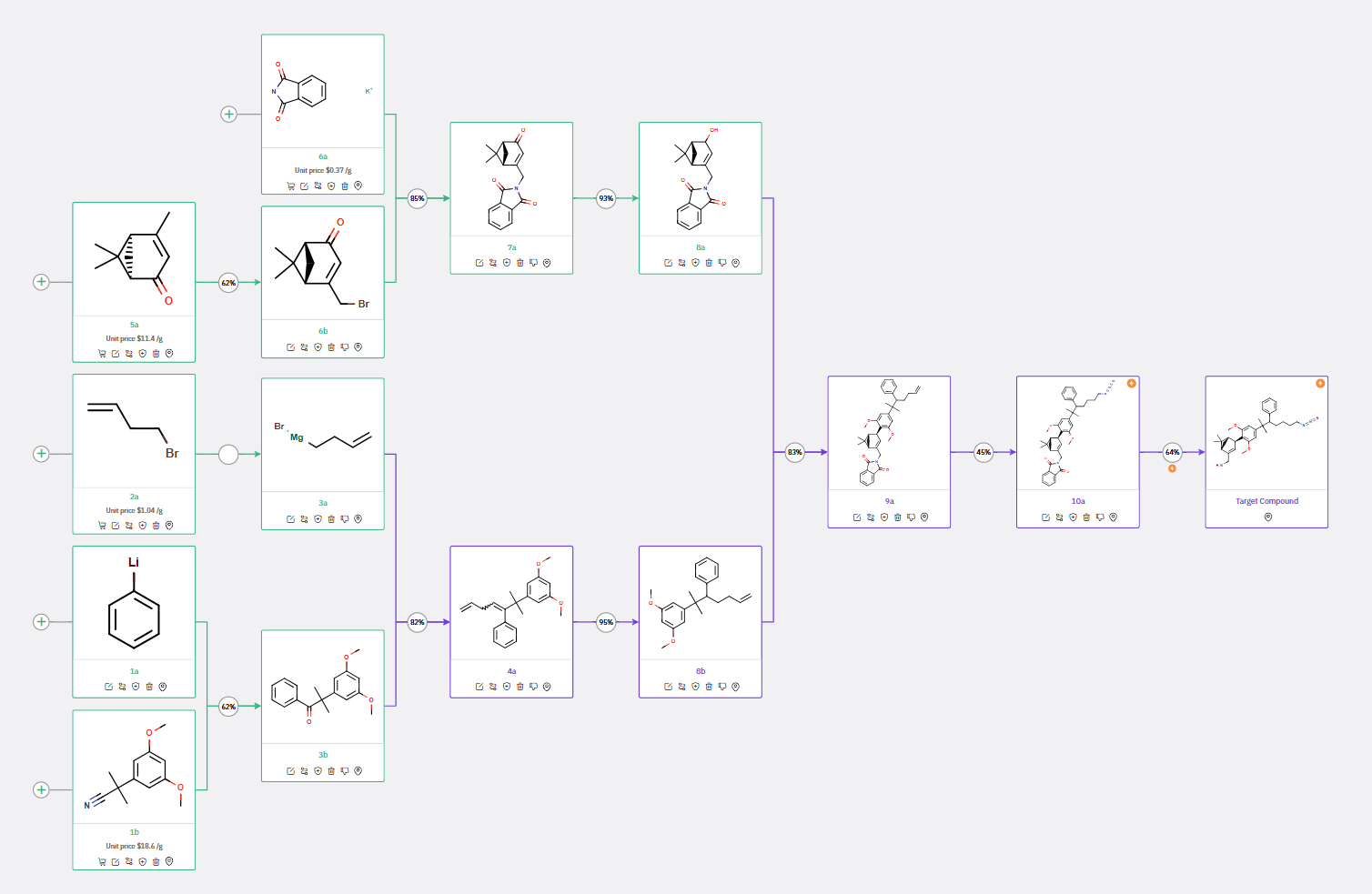
ChemAIRS focuses on the synthesis of these CB2R-selective inverse agonists, which are derived from the privileged scaffold of HU-308, a well-known CB2R agonist. The synthetic route commences with compound 3b, which was obtained via methylation of 3,5-dimethoxyphenylacetonitrile followed by treatment with phenyl lithium (Scheme 1).
In the subsequent step, ChemAIRS proposed using a Grignard reagent to introduce an alkyl side chain to the ketone 3b, yielding racemic compound 4a. The conjugated alkene 4a was selectively reduced through hole-transfer catalysis to furnish 8b, which then underwent Friedel-Crafts allylation with verbenol derivative 8a, producing intermediate 9a. Hydroazidation of the alkene with TMSN3 and subsequent phthalimide deprotection via hydrazine unveiled the target molecule.
Scheme 1: ChemAIRS predicted a synthetic pathway for CB2R inverse agonists
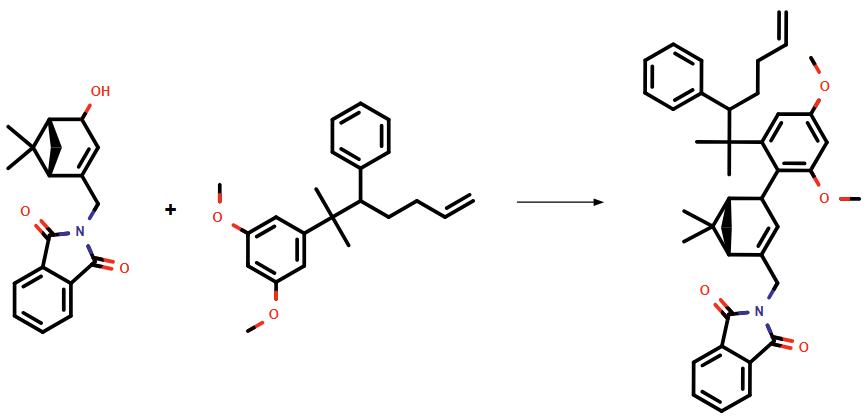
Moreover, our system identified a possible side reaction during the synthesis of 9a, as shown in Figure 1.
Figure 1: Potential side reaction for the synthesis of intermediate 9a
Alternative Synthetic Strategy for CB2R Inverse Agonist Development
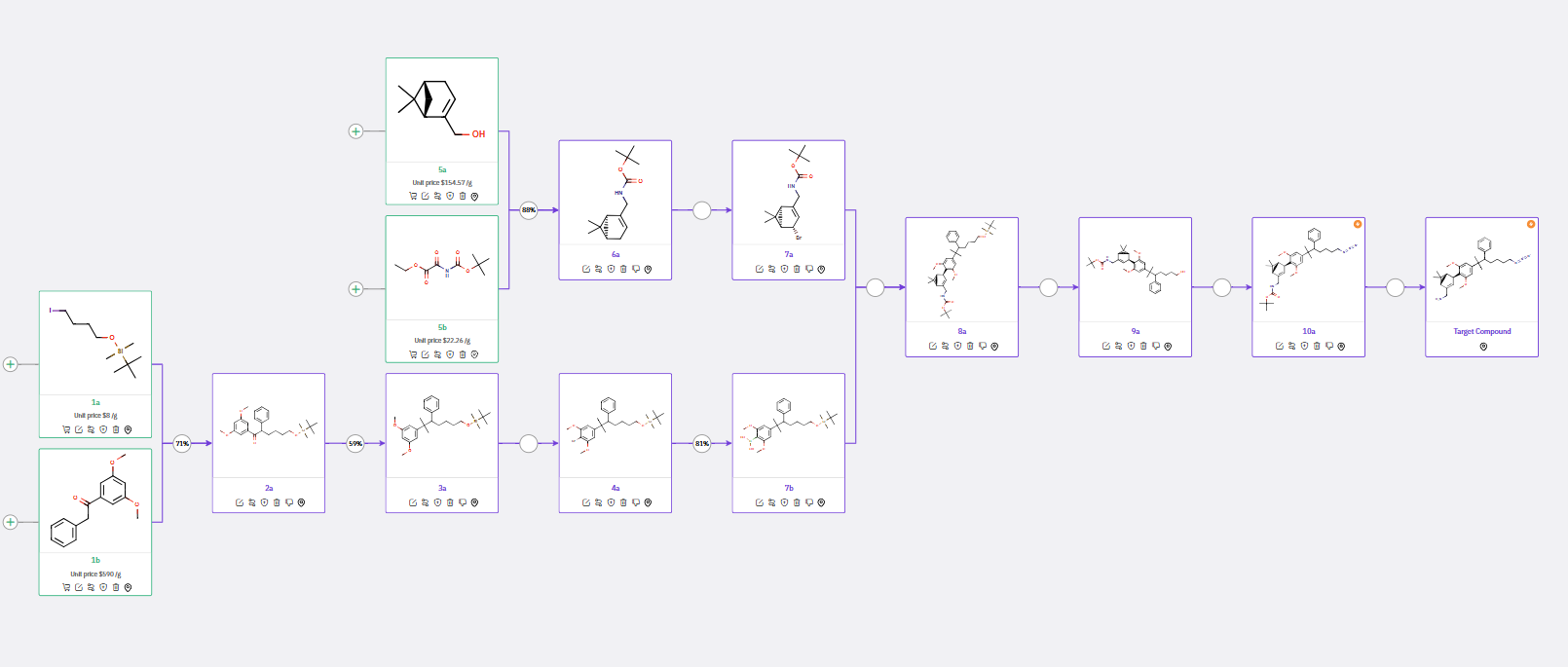
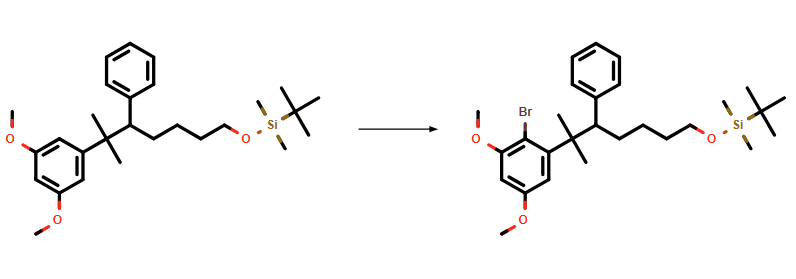
An alternative synthetic route is outlined in Scheme 2. Here, ChemAIRS suggested alkylating ketone 1b via reaction with iodide 1a. Introduction of the gem-dimethyl group was achieved by treatment of 2a with TiCl4 and AlMe3, affording 3a, which was then brominated and reacted with B(OMe)3 to yield arylboronic acid 7b.
In this alternative route, N-Boc-protected allylic amine 6a was formed under Mitsunobu conditions, and subsequent bromination yielded 7a. A Suzuki coupling between 7a and arylboronic acid 7b afforded 8a. The final step of this method involved Boc deprotection to generate the target molecule, in contrast to phthalimide removal as proposed in Scheme 1.
Scheme 2: Alternative synthetic route for CB2R inverse agonists
A potential side reaction during bromination was also identified, as depicted in Figure 2.
Figure 2: Potential side reaction for the synthesis of intermediate 4a
In conclusion, the development of CB2 inverse agonists presents a promising avenue for therapeutic intervention in inflammatory and immune-related diseases. The synthetic routes highlighted by ChemAIRS not only provide efficient methods for accessing these compounds but also reveal potential pitfalls in the synthesis, offering valuable insights for future optimization. Continued exploration of these pathways will enhance our understanding of CB2R modulation and its potential clinical applications.
Interested in exploring the capabilities of our Retrosynthesis module? Learn more here: ChemAIRS_Retrosynthesis