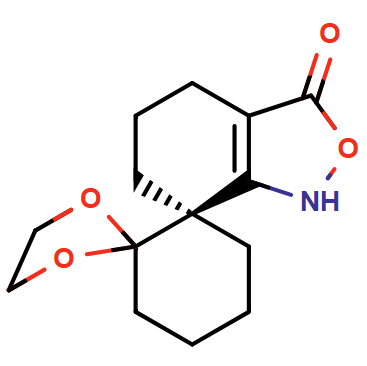
Leveraging ChemAIRS to Investigate Synthetic Strategies for Chiral Spirocyclic Isoxazolone, a Vital Building Block in a Boehringer Ingelheim Drug Development Program_EP08
Isoxazolines: Versatile Scaffolds with Enhanced Medicinal Properties
Isoxazolines, as five-membered heterocyclic scaffolds, exhibit diverse biological activities and are crucial intermediates in natural product synthesis. Their integration into complex organic compounds is particularly appealing in medicinal chemistry due to their potential anti-inflammatory, antibacterial, antifungal, and anticancer properties.
Additionally, the inclusion of spirocyclic carbon centers enhances spatial rigidity, which can improve the physicochemical properties and pharmacokinetic profiles of drug candidates.
Reference: https://pubs.acs.org/doi/10.1021/acs.oprd.4c00008?ref=PDF
Synthetic Strategies for Chiral Spirocyclic Isoxazolone: ChemAIRS-Assisted Route Development
In this study, we employed our ChemAIRS platform to propose synthetic routes for a chiral spirocyclic isoxazolone, a crucial building block in a drug development program at Boehringer Ingelheim.
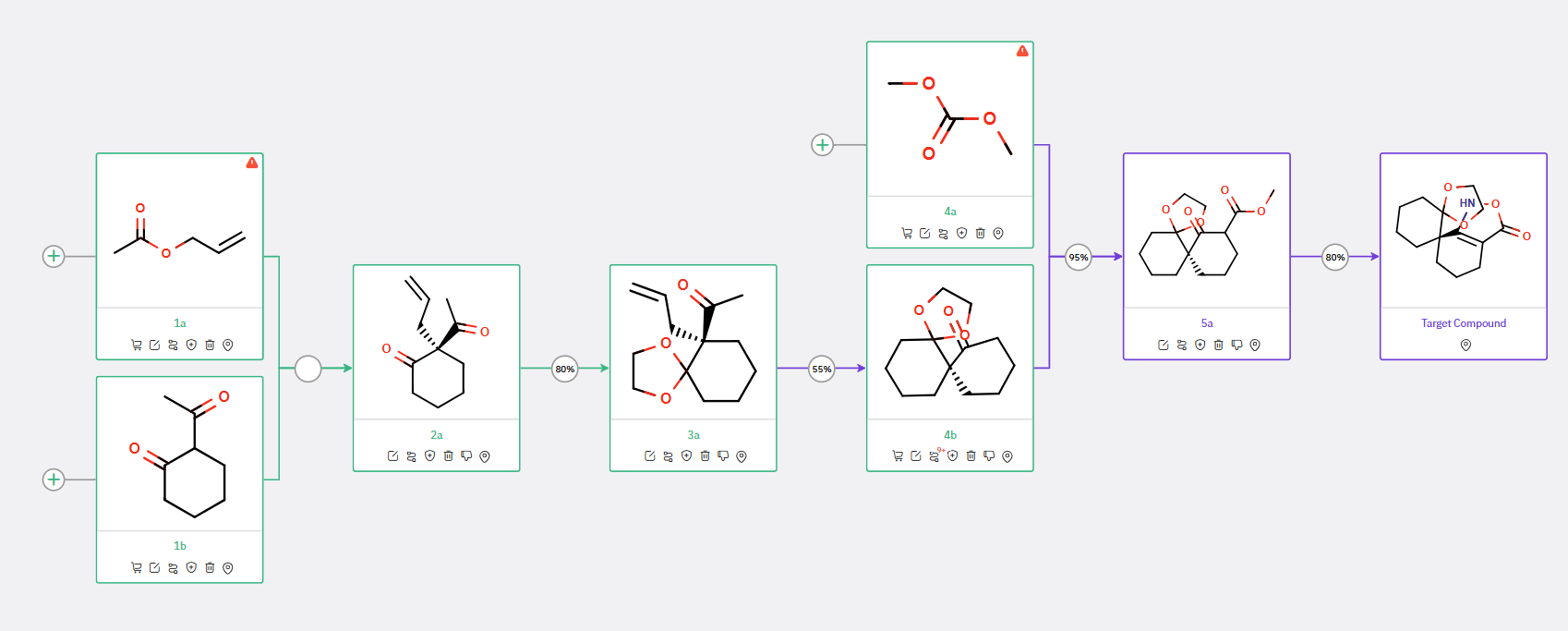
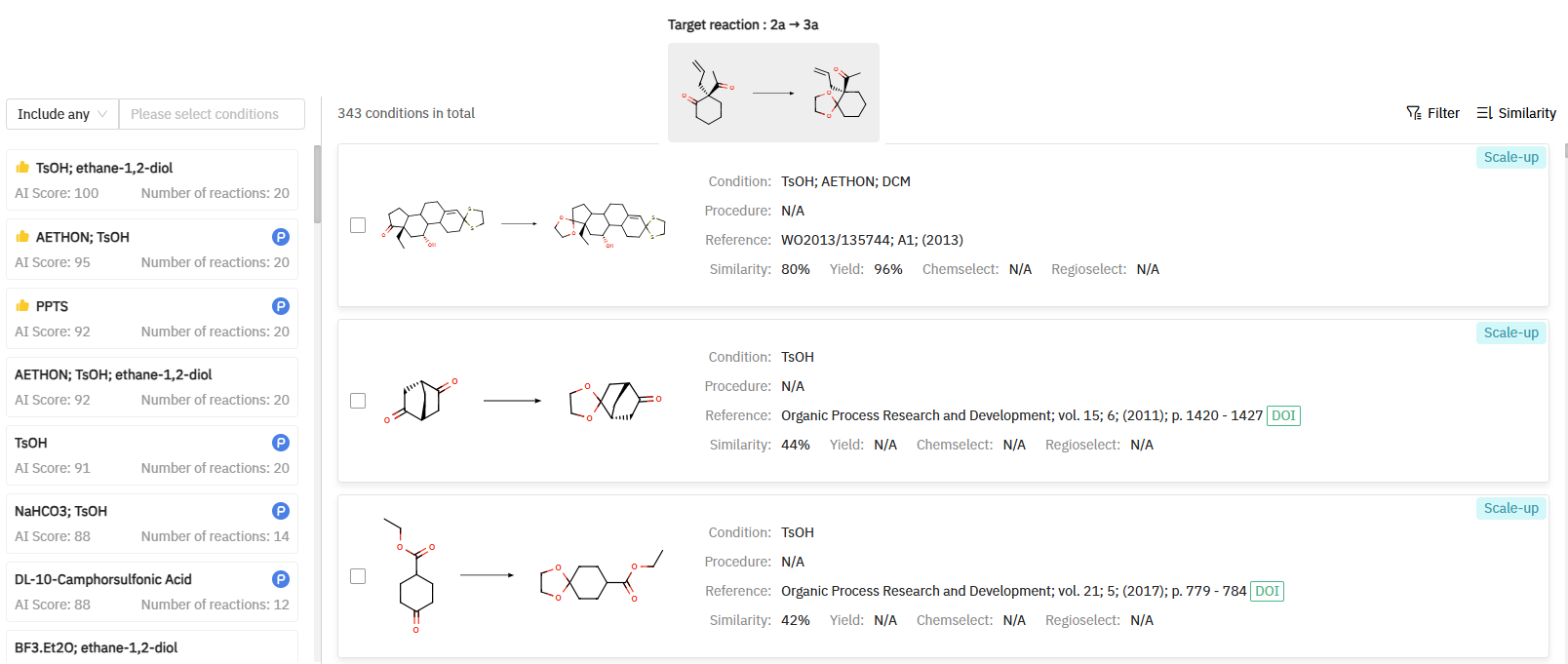
The first synthetic strategy involves the preparation of a chiral intermediate (compound 2a in Scheme 1) through a catalytic asymmetric allylation of commercially available diketones 1b. The resulting cyclohexanone derivative 2a is subsequently protected as a ketal (3a) to mitigate potential side reactions in the following steps. ChemAIRS can suggest scale-up conditions for this reaction (Figure 1).
Interested in exploring the capabilities of our Process Chemistry module? Learn more here: ChemAIRS_Process Chemistry
Scheme 1: ChemAIRS predicted a synthetic pathway for spirocyclic isoxazolone, starting from a commercially available starting material 1b
Figure 1: ChemAIRS’ suggested reaction conditions for scaling up intermediate 3a
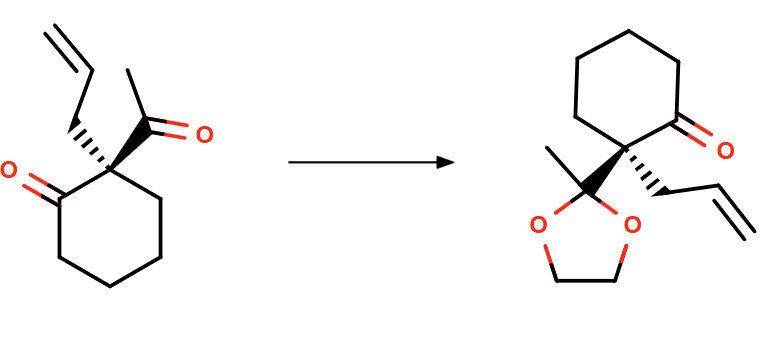
Additionally, our platform can predict possible side reactions during the synthesis of intermediate 3a (as depicted in Figure 2). The spirocyclohexane precursor 4b can subsequently be formed through an intramolecular hydroalkylation catalyzed by a palladium catalyst system.
Figure 2: Potential side reaction for the synthesis of 3a
Alternative Route to Chiral Spirocyclic Isoxazolone
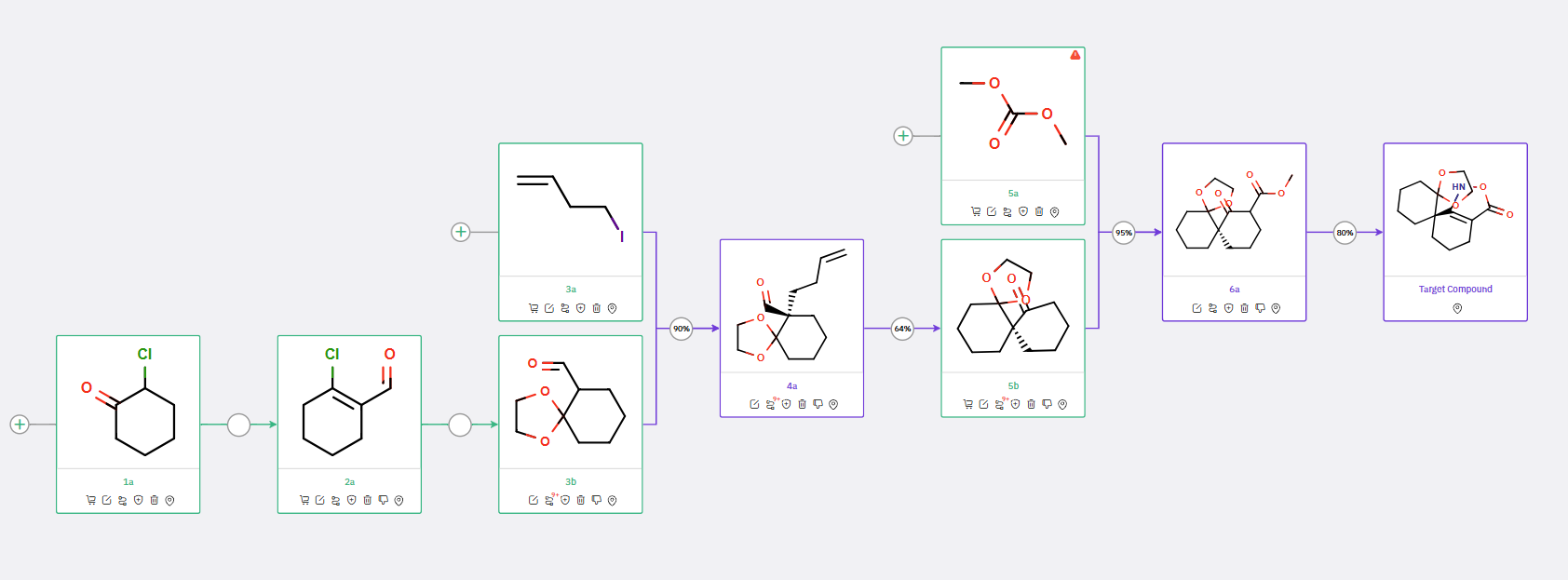
ChemAIRS also identified an alternative synthetic route to the chiral spirocyclic isoxazolone, starting from different commercially available starting materials (compound 1a in Scheme 2). In this route, the intermediate 4a is synthesized via a three-step procedure, with the key chirality-inducing allylation step potentially yielding a mixture of isomers that could be resolved through chiral chromatography.
Scheme 2: Alternative synthetic route for spirocyclic isoxazolone
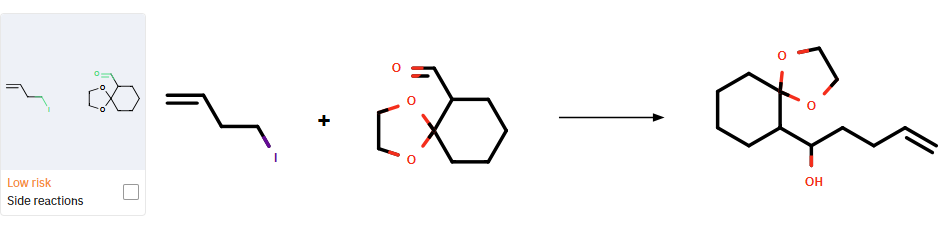
Furthermore, a side reaction is detected during the synthesis of compound 4a (Figure 3).
Figure 3: Potential side reaction for the synthesis of 4a
In conclusion, ChemAIRS successfully provided multiple synthetic pathways for a key intermediate in medicinal chemistry, offering flexibility in the selection of starting materials. Additionally, the platform can identify potential side reactions and recommend scalable reaction conditions.