Utilizing ChemAIRS to Explore Synthetic Strategies of Azabenzimidazolone: A Potent αvβ1 Integrin Antifibrotic by Takeda_07
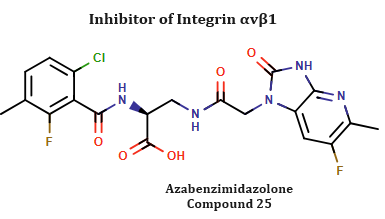
Azabenzimidazolone as a Potent Antifibrotic Agent Targeting αvβ1 Integrin
Selective inhibition of the RGD (Arg-Gly-Asp) integrin αvβ1 has gained traction as a therapeutic approach for liver fibrosis, owing to the integrin's critical role in disease progression, target specificity, and favorable safety profile.
Researchers at Takeda Pharmaceuticals have recently identified Azabenzimidazolone (referred to as compound 25 in their study) as a potent antifibrotic agent in an in vivo rat liver model. This compound serves as a valuable tool for probing selective αvβ1 inhibition.
Reference: https://pubs.acs.org/doi/10.1021/acs.jmedchem.4c00743?ref=PDF
ChemAIRS Predicts a Synthetic Pathway for Azabenzimidazolone
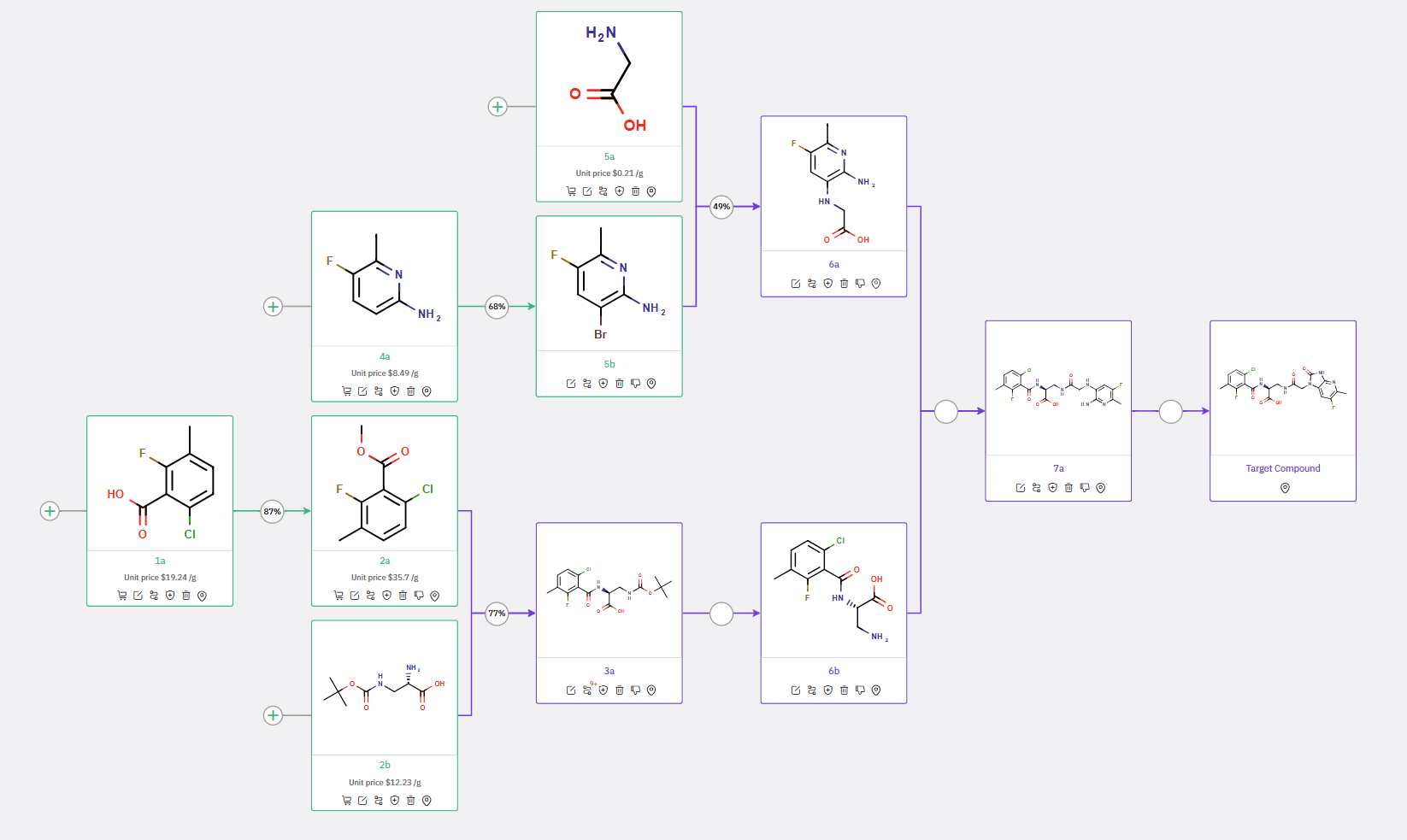
Upon submission to ChemAIRS, a 7-step synthetic route to Azabenzimidazolone was rapidly proposed, diverging significantly from the methodology reported by Takeda (Scheme 1). The synthesis begins with the formation of two key intermediates: compound 6a (synthesized via a C,N-cross coupling reaction between precursors 5a and 5b), and compound 6b. These intermediates then undergo an amide coupling, followed by a ring-closing reaction to yield the final API. Notably, ChemAIRS suggested performing the ring-closing reaction as the terminal step in the synthesis, in contrast to Takeda's strategy.
For the synthesis of intermediate 6b, ChemAIRS proposed an alternative to the literature-reported method, which involved protected building blocks such as diaminoester. Instead, ChemAIRS recommended using Boc-amino alanine (compound 2b), with the proposed pathway involving an amidation between compounds 2a and 2b to produce compound 3a, followed by Boc-deprotection to yield compound 6b.
Scheme 1: ChemAIRS proposed a synthetic pathway for Azabenzimidazolone, recommending the ring-closing reaction as the final step
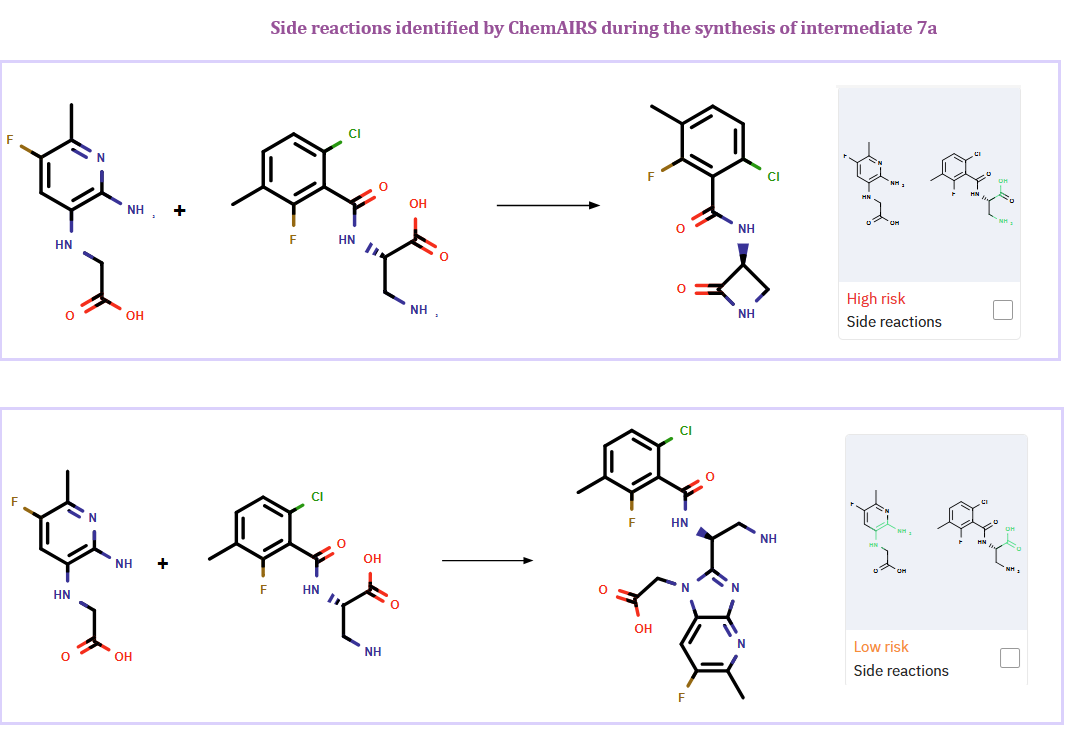
Additionally, ChemAIRS identified potential side reactions during the synthesis of intermediate 7a, as depicted in Figure 1.
Figure 1: ChemAIRS detected potential side reactions during the synthesis of intermediate 7a
Proposing Alternative Ring-Closing Strategy for Azabenzimidazolone
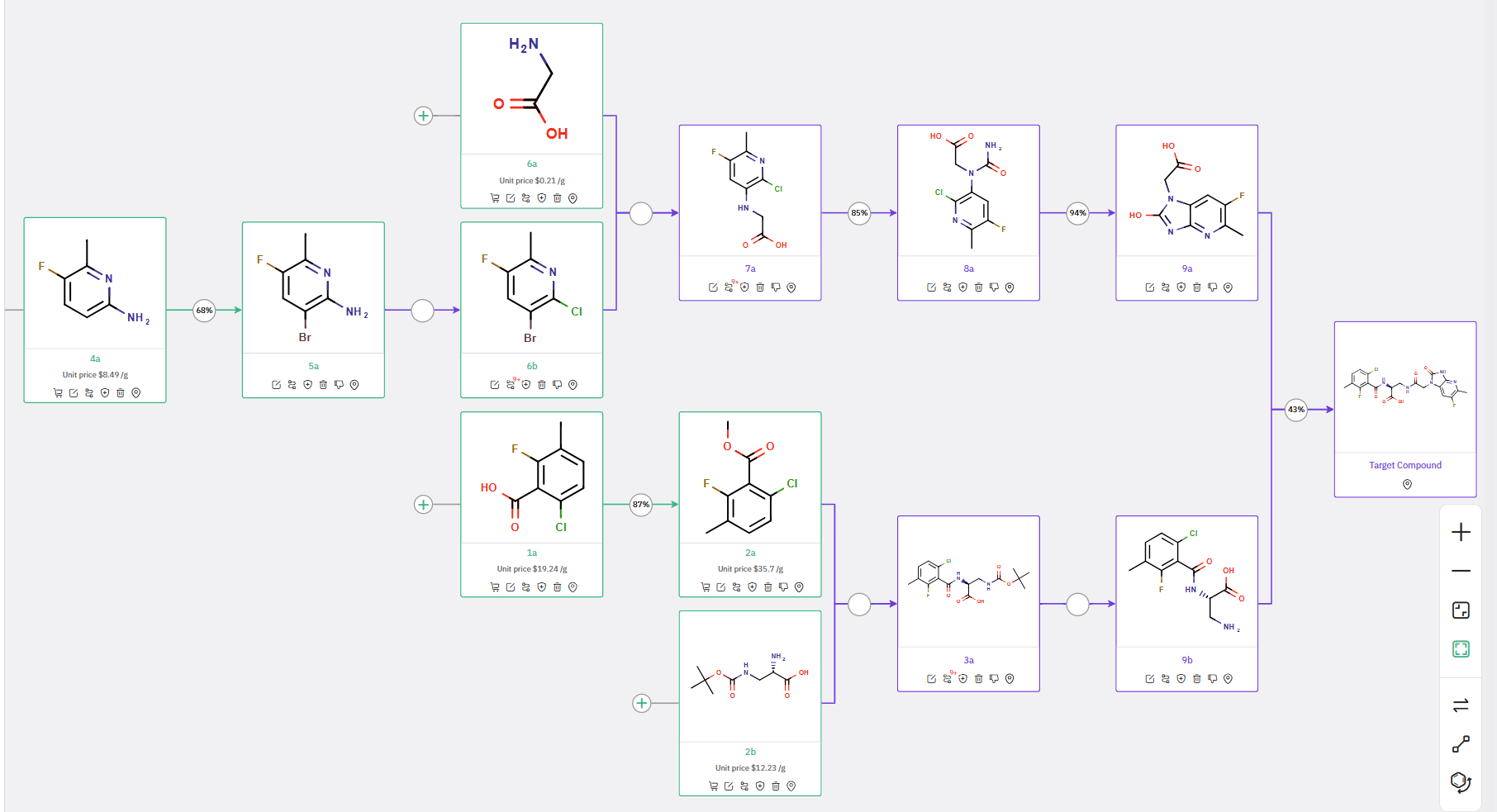
In addition to the primary route, ChemAIRS also offered an alternative synthesis for the target molecule, as detailed in Scheme 2. In this alternative strategy, the ring-closing step to form compound 9a is proposed to occur before the final amide coupling of 9a and 9b.
Scheme 2: Alternative Synthetic Route for Azabenzimidazolone suggested by ChemAIRS
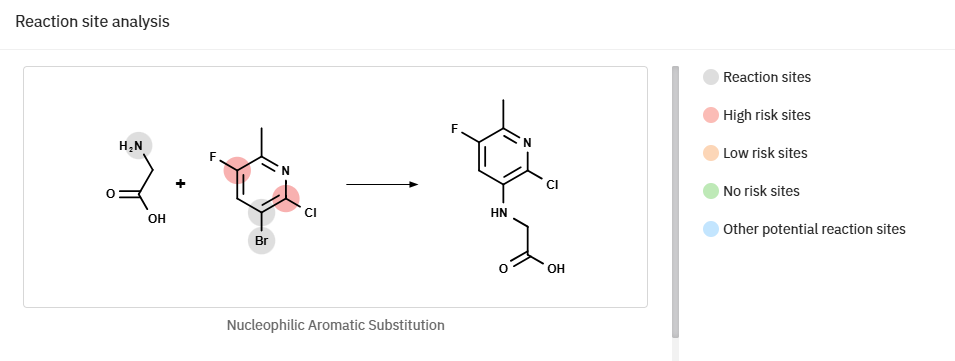
Furthermore, ChemAIRS detected possible side reactions during the synthesis of intermediate 7a, where either Cl or F of compound 6b could be substituted (Figure 2).
Figure 2: ChemAIRS reaction site analysis for the synthesis of intermediate 7a
ChemAIRS demonstrates its capability to innovate by proposing distinct synthetic routes that differ from established methodologies. Additionally, its ability to identify potential side reactions and suggest alternative pathways underscores its reliability and advanced problem-solving capabilities in synthetic organic chemistry.
Interested in exploring the capabilities of our Retrosynthesis module? Learn more here: ChemAIRS_Retrosynthesis