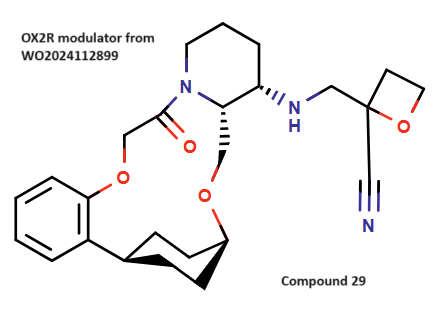
Use ChemAIRS to Investigate Synthesis Strategies of OX2R Modulator, a Vertex Pharmaceuticals' Approach to Narcolepsy Treatment_EP06
Macrocyclic Amine Modulators Targeting OX2R: A Promising Narcolepsy Treatment
Orexins, a class of neuropeptides, influence sleep/wake cycles through interaction with G-protein-coupled receptors OX1R and OX2R. Research suggests that OX2R agonists may be promising therapeutic agents for narcolepsy. Vertex Pharmaceuticals has designed macrocyclic amine modulators with high affinity and specificity for OX2R, demonstrating enhanced CNS penetration and favorable pharmacokinetic profiles.
Reference:
https://patentscope.wipo.int/search/de/detail.jsf?docId=WO2024112899&_cid=P10-LWUD65-81974-1
Replication of the Reported Synthesis Route of OX2R Modulator
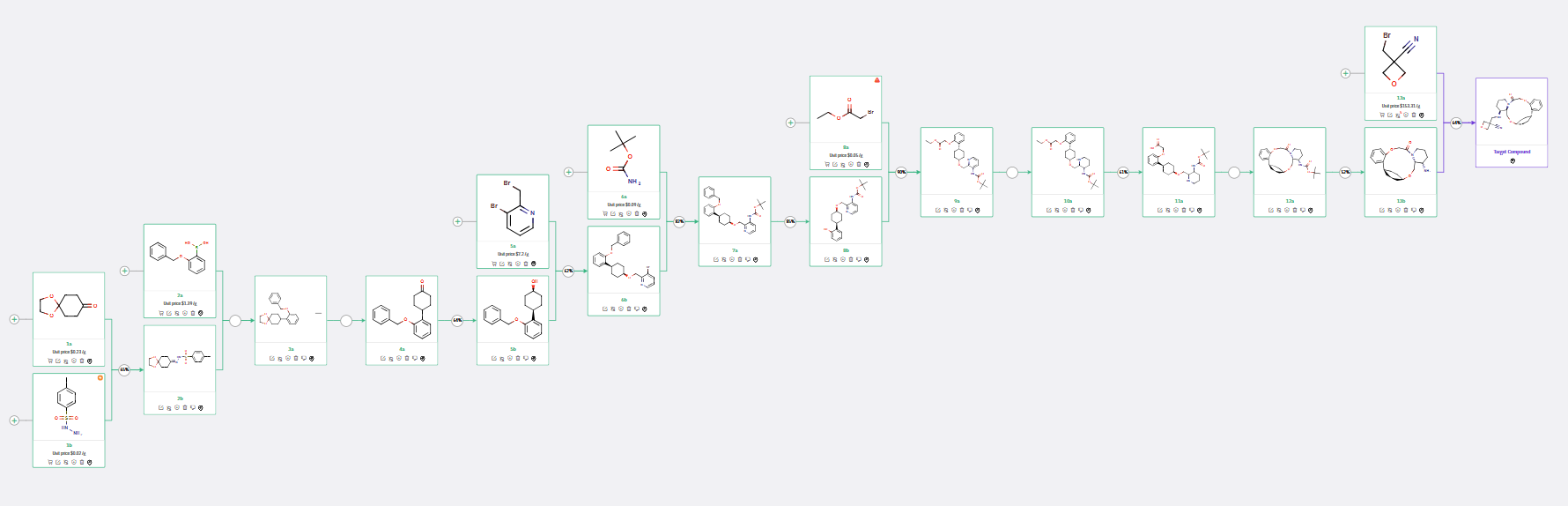
ChemAIRS examined the synthesis of an OX2R modulator, specifically focusing on compound 29 from Vertex’s patent. The synthesis involved two key intermediates, 13a and 13b, with 13a being commercially sourced (Scheme 1). The pathway to 13b closely follows the method described by Vertex Pharmaceuticals, where the precursor cyclohexanol 5b was stereoselectively obtained via ketone 4a reduction. A crucial chirality-inducing step included the reduction of Boc-amino pyridine (9a) to Boc-amino piperidine (10a), producing a racemic mixture subsequently resolved via chiral chromatography.
In the final synthetic step, ChemAIRS recommended a direct one-step synthesis to the final compound using the commercially available 3-(bromomethyl)oxetane-3-carbonitrile (13a), in contrast to a previous three-step process starting from 3-(hydroxymethyl)oxetane-3-carbonitrile.
Scheme 1: ChemAIRS predicted a synthetic pathway for an OX2R modulator, which closely aligns with Vertex's patented report
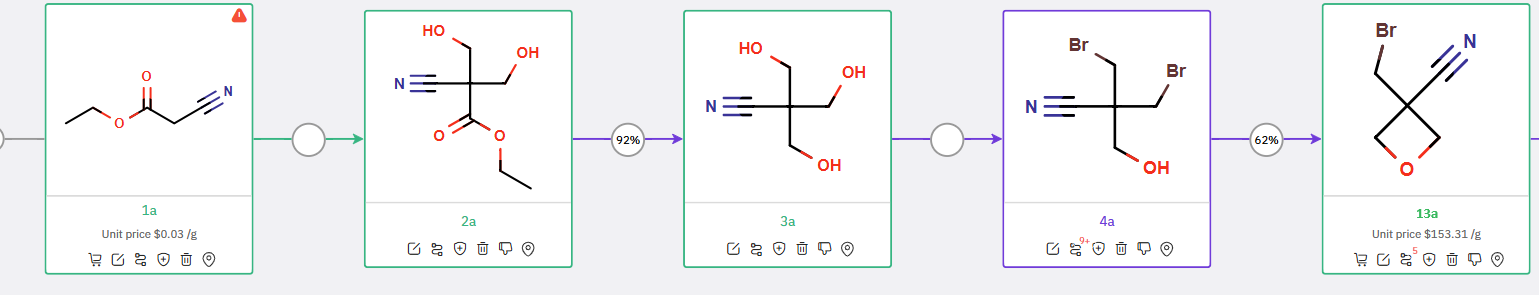
Additionally, to offer a more economical path to obtain 13a, ChemAIRS proposed a cost-effective synthetic route for 13a as depicted in Scheme 2.
Scheme 2: Proposed synthetic strategy for 13a from ChemAIRS
Proposing Alternative Synthetic Strategies for OX2R Modulator
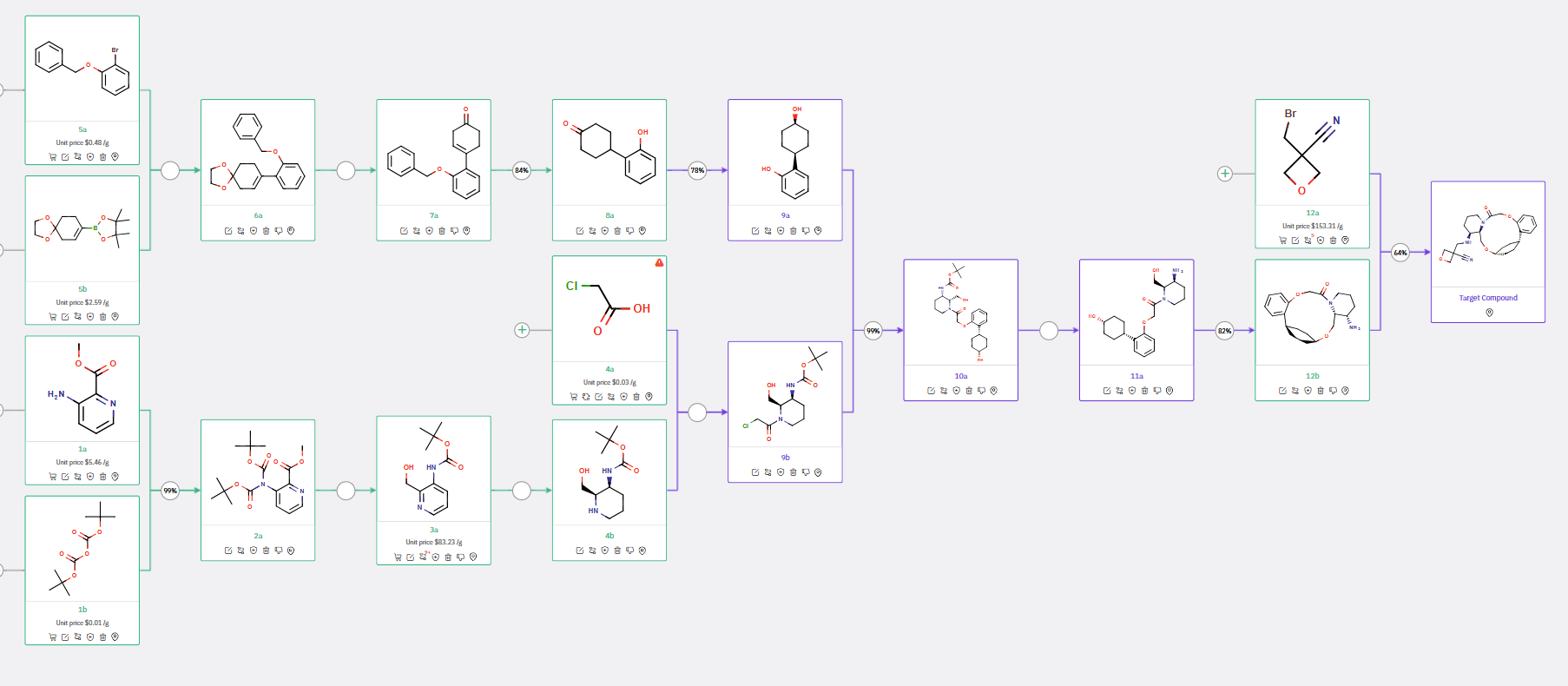
ChemAIRS not only identified a synthetic route similar to previously reported methods but also proposed alternative strategies for synthesizing the final API, as illustrated in Schemes 3 and 4.
Scheme 3 outlines the synthesis of two chiral molecules, 9a and 9b, from commercially available precursors, which are then condensed to form 10a and further processed to yield the key intermediate 12b.
Interested in exploring the capabilities of our Retrosynthesis module? Learn more here: ChemAIRS_Retrosynthesis
Scheme 3: Alternative Synthetic Routes for the OX2R Modulator, initiated with the preparation of two chiral intermediates
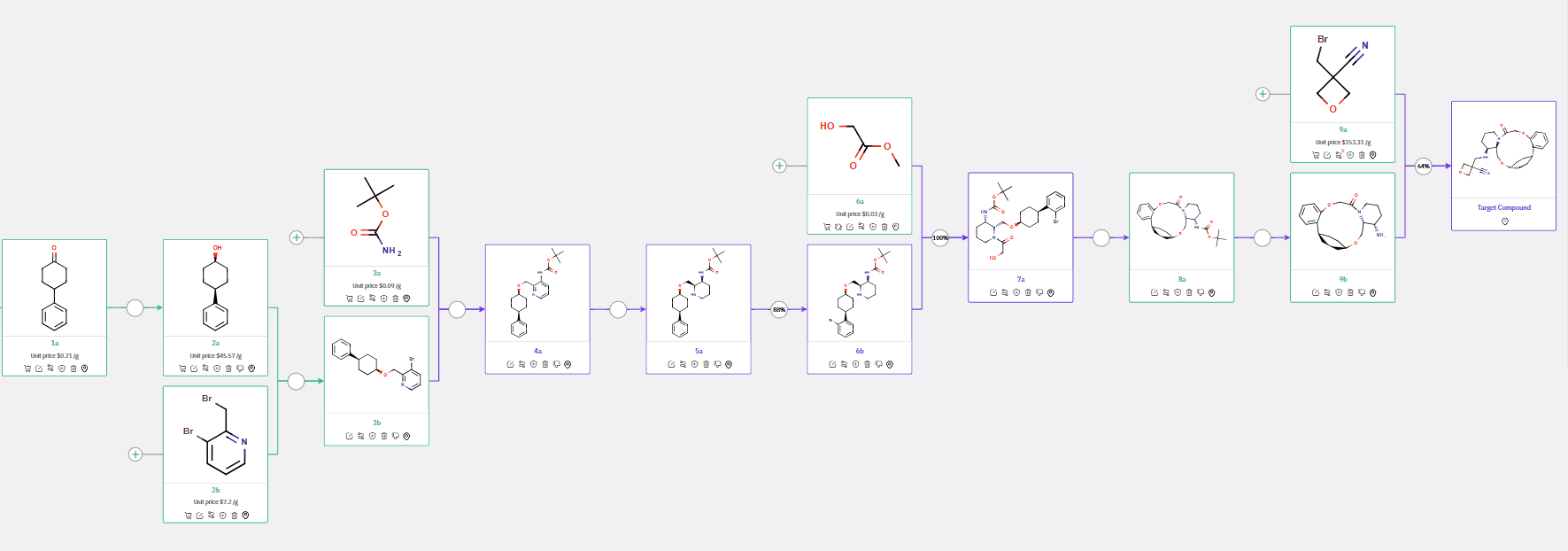
Scheme 4 describes the synthesis of chiral 4-phenylcyclohexanol 2a in a single step, followed by its reaction with bromo-pyridine 2b and subsequent reduction of an additional aromatic ring, leading to the formation of chiral piperidinyl carbamate 5a.
ChemAIRS also suggested various strategies for synthesizing a macrocyclic molecule, such as the condensation of 11a to produce 12b (Scheme 3) or the intramolecular etherification of 7a to yield 8a (Scheme 4).
Scheme 4: Alternative Synthetic Routes for OX2R Modulator, featuring the formation of the macrocyclic molecule through intramolecular etherification
In summary, ChemAIRS excels in providing synthetic pathways consistent with established methodologies and proposing innovative, practical alternatives. This versatility enables chemists to explore new synthetic avenues, facilitating accelerated innovation in the lab and positioning ChemAIRS as a valuable resource for advancing chemical research and development