Rapidly Identifying Alternative Synthetic Routes for Merck's KRAS G12C Inhibitor_EP04
Merck's Discovery of Selective G12C Inhibitors for KRAS-Mutant Cancers
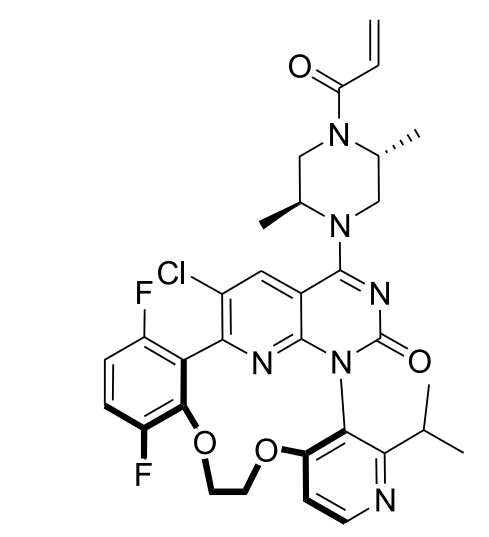
Cancer is the second leading cause of death globally and a major health challenge. The KRAS (Kirsten rat sarcoma) oncogene is frequently mutated in various cancers, causing resistance to standard treatments and poor outcomes. These mutations hyperactivate cell proliferation, thus driving tumor growth. Merck researchers have discovered heteroaryl compounds that selectively inhibit the G12C mutant KRAS protein, offering a promising therapeutic approach.
Reference: https://pubs.acs.org/doi/10.1021/acsmedchemlett.1c00389?ref=PDF
ChemAIRS Predicted the Reported Synthetic Route for the Macrocyclic Compound
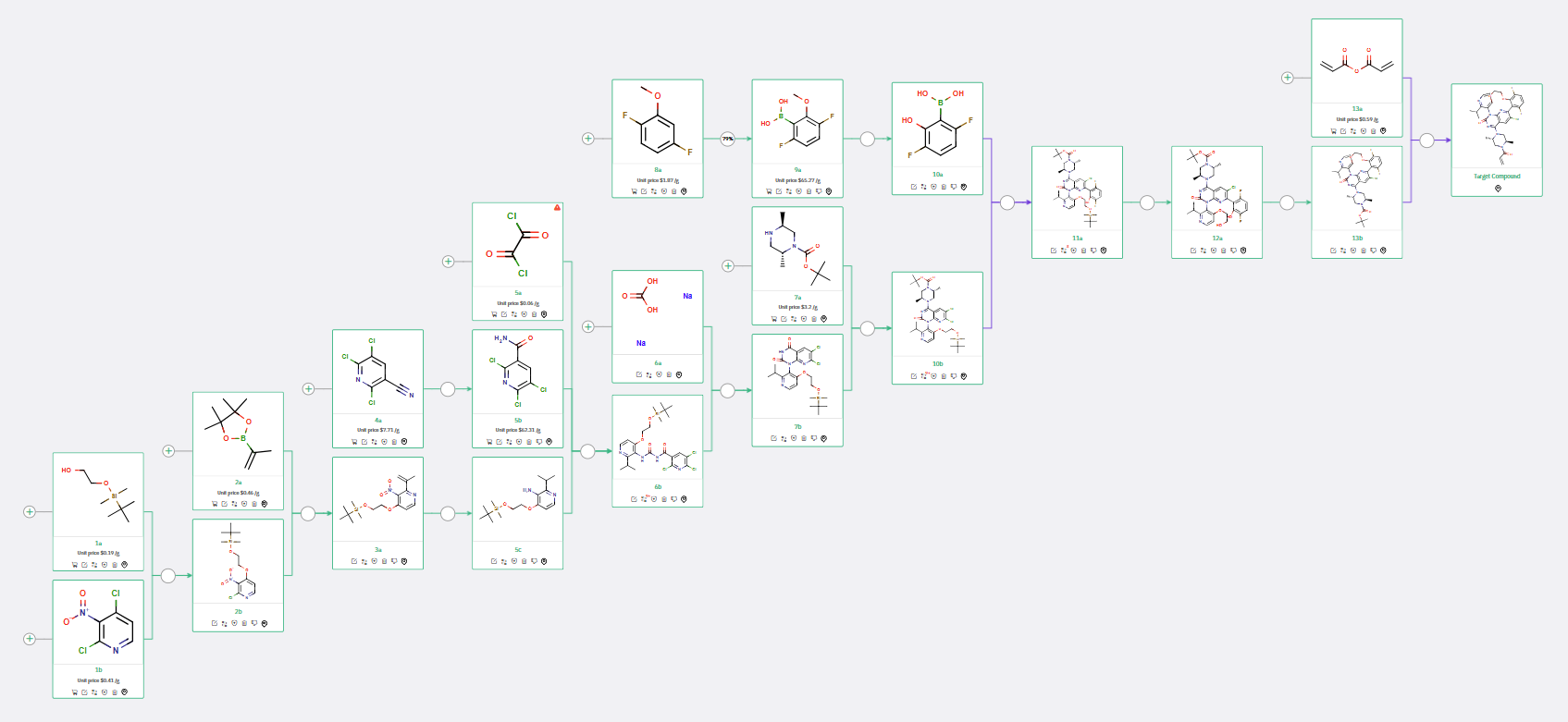
ChemAIRS generated multiple synthetic pathways to the target macrocyclic molecule. One of these pathways (Scheme 1) closely aligned with the synthesis recently reported by Merck. Additionally, our retrosynthesis platform proposed an alternative synthetic route for compound 11a, starting from boronic acid 10 and proceeding via a 2-step process, as opposed to the 3-step synthesis from potassium (3,6-difluoro-2-hydroxyphenyl) trifluoroborate found in the literature. In the final step, the system suggested an alternative reaction condition using acrylic anhydride and methylmorpholine instead of the reported acryloyl chloride/DIPEA combination.
Scheme 1: ChemAIRS predicted a synthetic pathway for Merck's KRAS inhibitor, closely aligned with the published route
ChemAIRS as a Retrosynthesis Tool Enhances Route Planning and Risk Detection
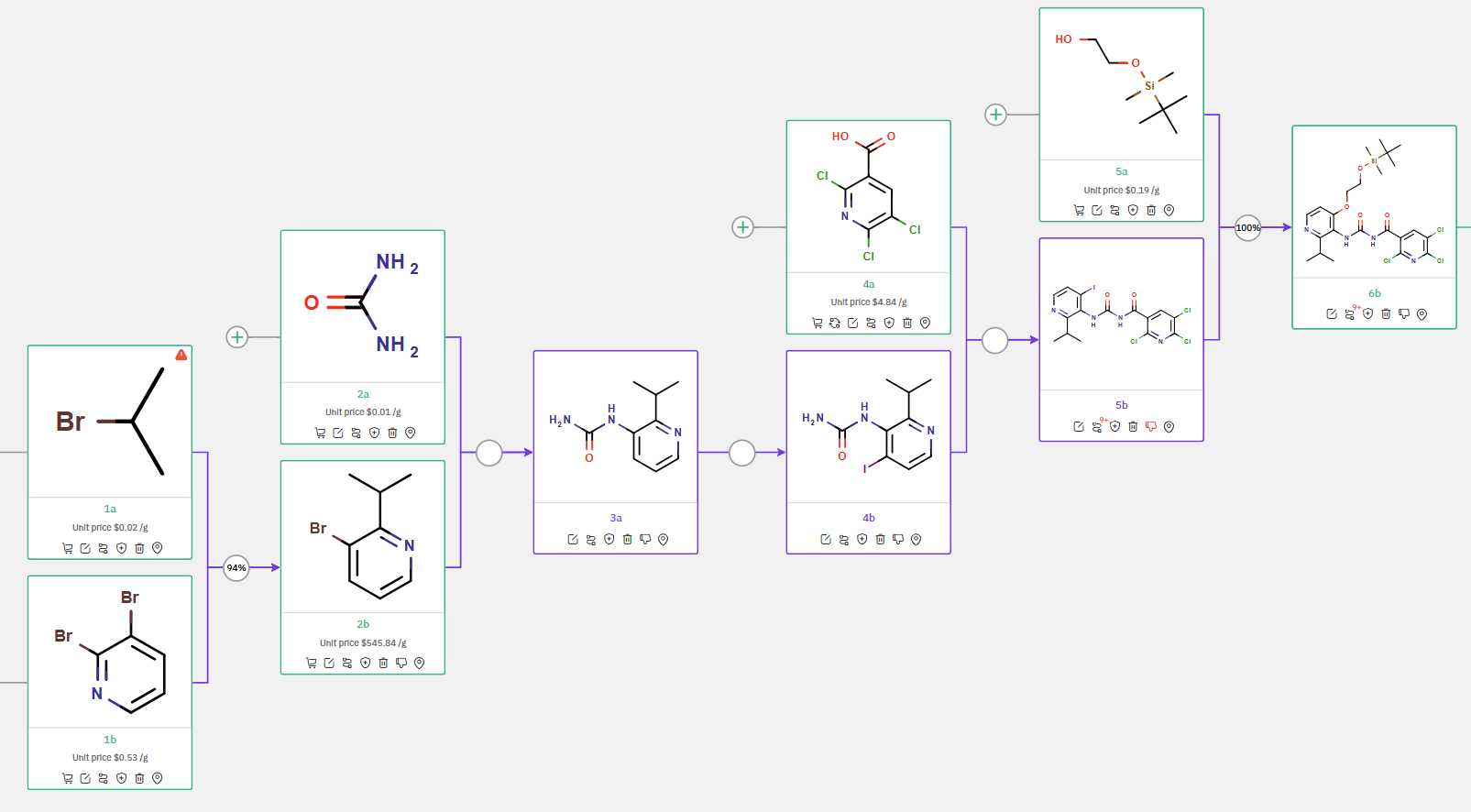
Another synthetic route for compound 6b was proposed in Scheme 2. Monoarylurea derivative 3a, produced from the reaction of bromo isopropylpyridine 2b and urea 2a, was transformed into iodo-arylurea 4b, which then reacted with 4a to yield carbonylurea 5b. Subsequent alkoxylation of 5b with tert-butyldimethylsilyloxyethanol 5a led to the formation of compound 6b.
Scheme 2: ChemAIRS suggested an alternative synthetic route for 6b, a key intermediate for route optimization.
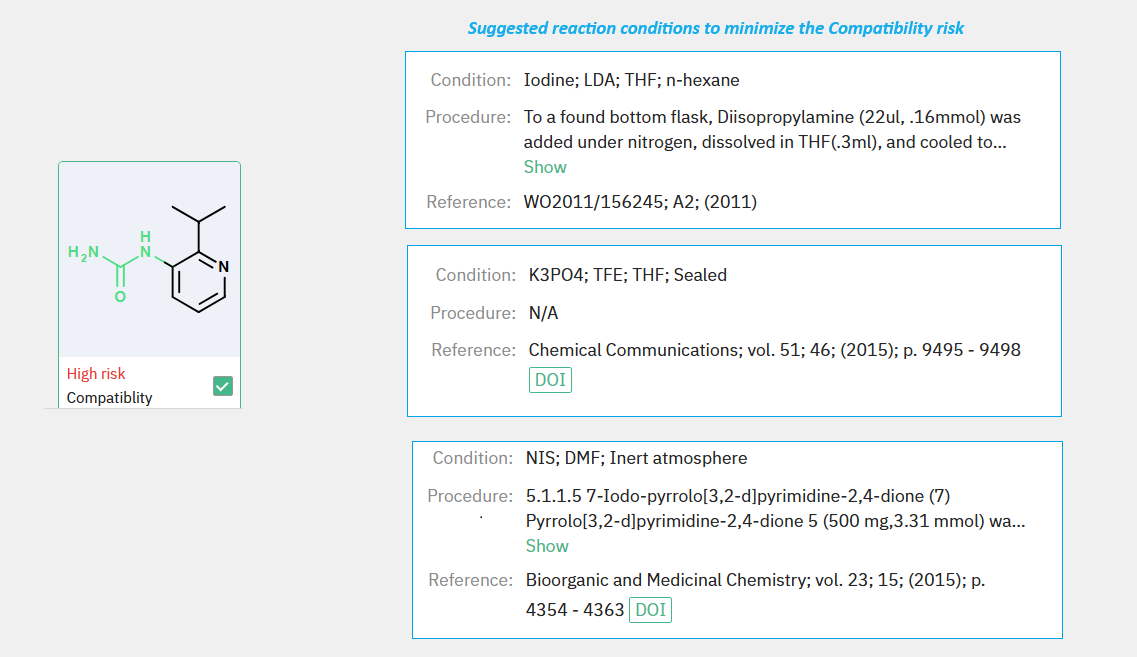
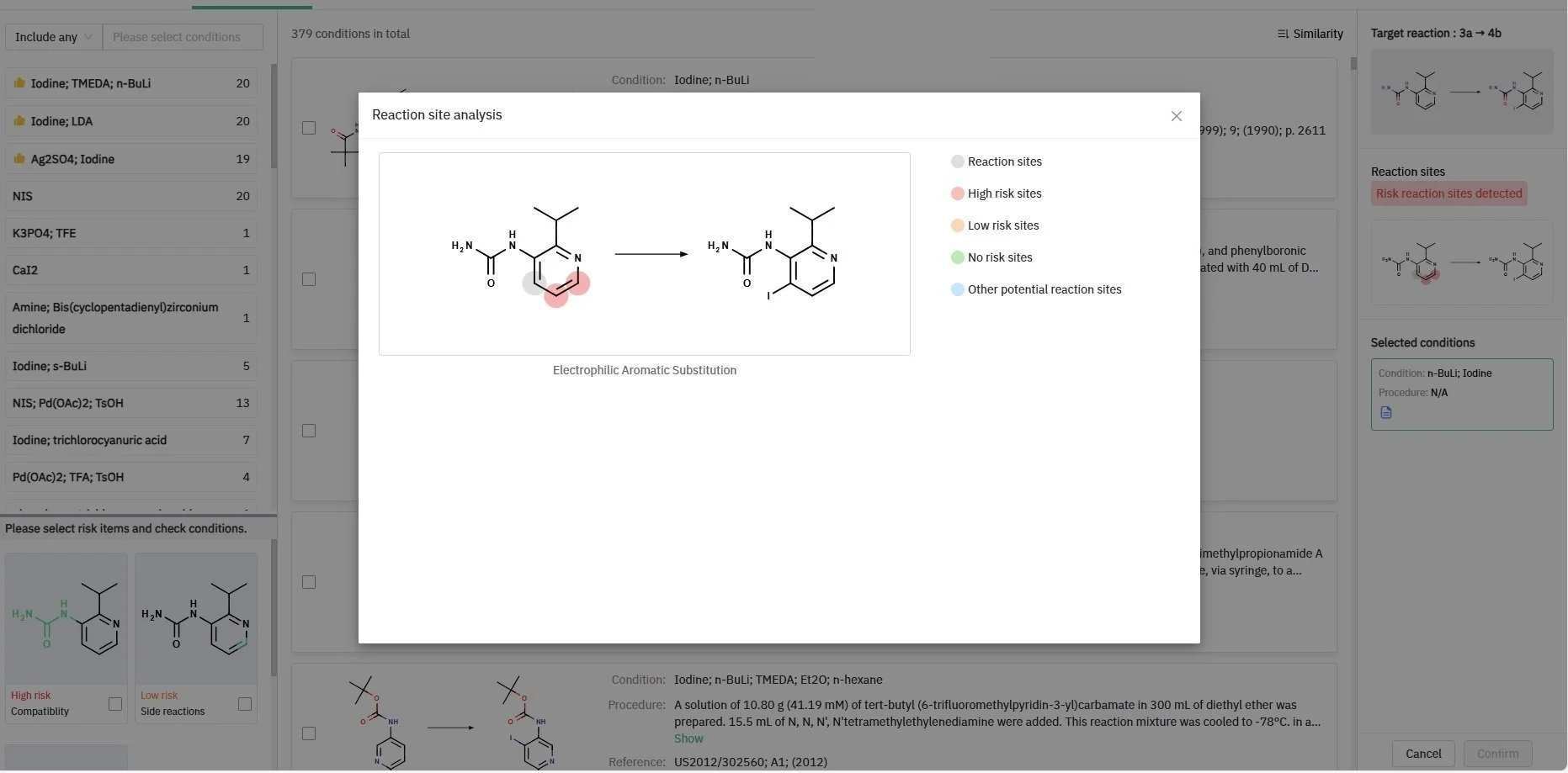
A key feature of our retrosynthesis tool is its ability to flag potential issues with functional group compatibility or side reactions. For instance, during the synthesis of compound 4b, the system identified a high-risk alert concerning the urea moiety of precursor 3a. Accordingly, our program recommended alternative conditions that accommodate the sensitivity of this functional group (Figure 1). Moreover, the system pinpointed risk detection sites specific to this synthesis step (Figure 2).
Interested in exploring the capabilities of our Retrosynthesis module? Learn more here: ChemAIRS_Retrosynthesis
Figure 1: ChemAIRS identified functional group compatibility risk for compound 3a and suggested alternative reaction conditions
Figure 2: ChemAIRS analyzed the reaction site for compound 3a to advise users on potential side reactions
In conclusion, ChemAIRS efficiently proposed synthetic routes for KRAS G12C inhibitors, aligning with known methods and suggesting more efficient alternatives. Additionally, the retrosynthesis tool's ability to detect and address potential risks further ensures the reliability and safety of the proposed synthetic pathways.