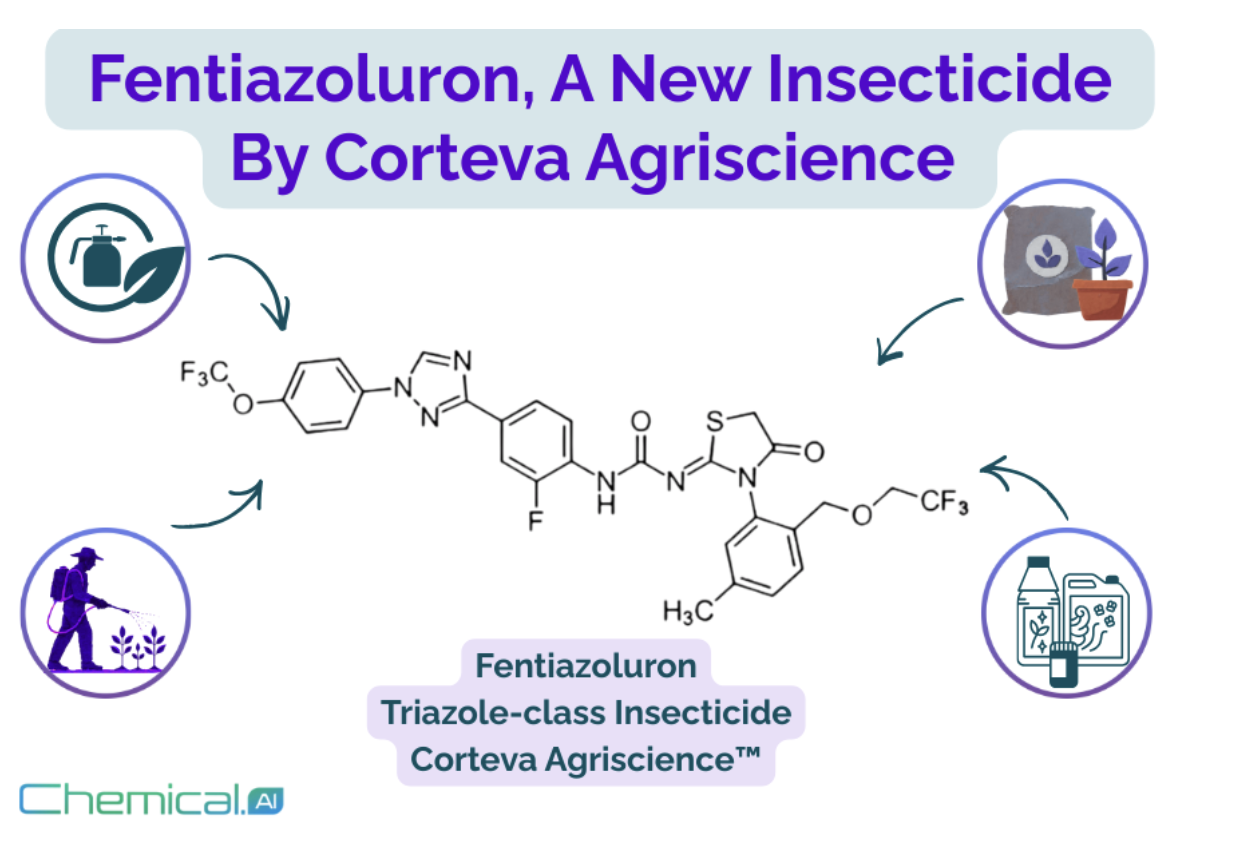
Corteva’s New Insecticide Receives ISO Name: A New Member Joins the Agrochemical Industry
In September 2025, the ISO Technical Committee on Pesticide Common Names officially granted the name fentiazoluron to a new insecticide developed by Corteva Agriscience. With this decision, the compound received an international provisional common name - essentially a global “passport” that marks an important step from laboratory research toward large-scale field use and commercialization.
R&D Background
Fentiazoluron was developed by Corteva Agriscience, an agricultural technology company spun off from DowDuPont and now a major player in the global agrochemical industry.
Corteva invests heavily in innovation to address growing challenges such as pest resistance, environmental pressure, and changing regulatory requirements. While triazole compounds are already well known in agriculture, mainly as fungicides, Corteva’s expansion of this chemical framework into insecticides highlights its ability to adapt proven chemistry to new biological targets.
Potential Properties
Although full biological data have not yet been released, the molecular structure suggests several promising features. The presence of multiple fluorinated groups may enhance insecticidal potency and environmental stability, while the triazole ring hints at a potentially novel mode of action affecting key insect physiological processes.
ChemAIRS Route Design
Synthesizing a molecule of this complexity requires careful and flexible route planning. Using ChemAIRS Retrosynthesis, multiple viable synthetic pathways were designed for fentiazoluron.
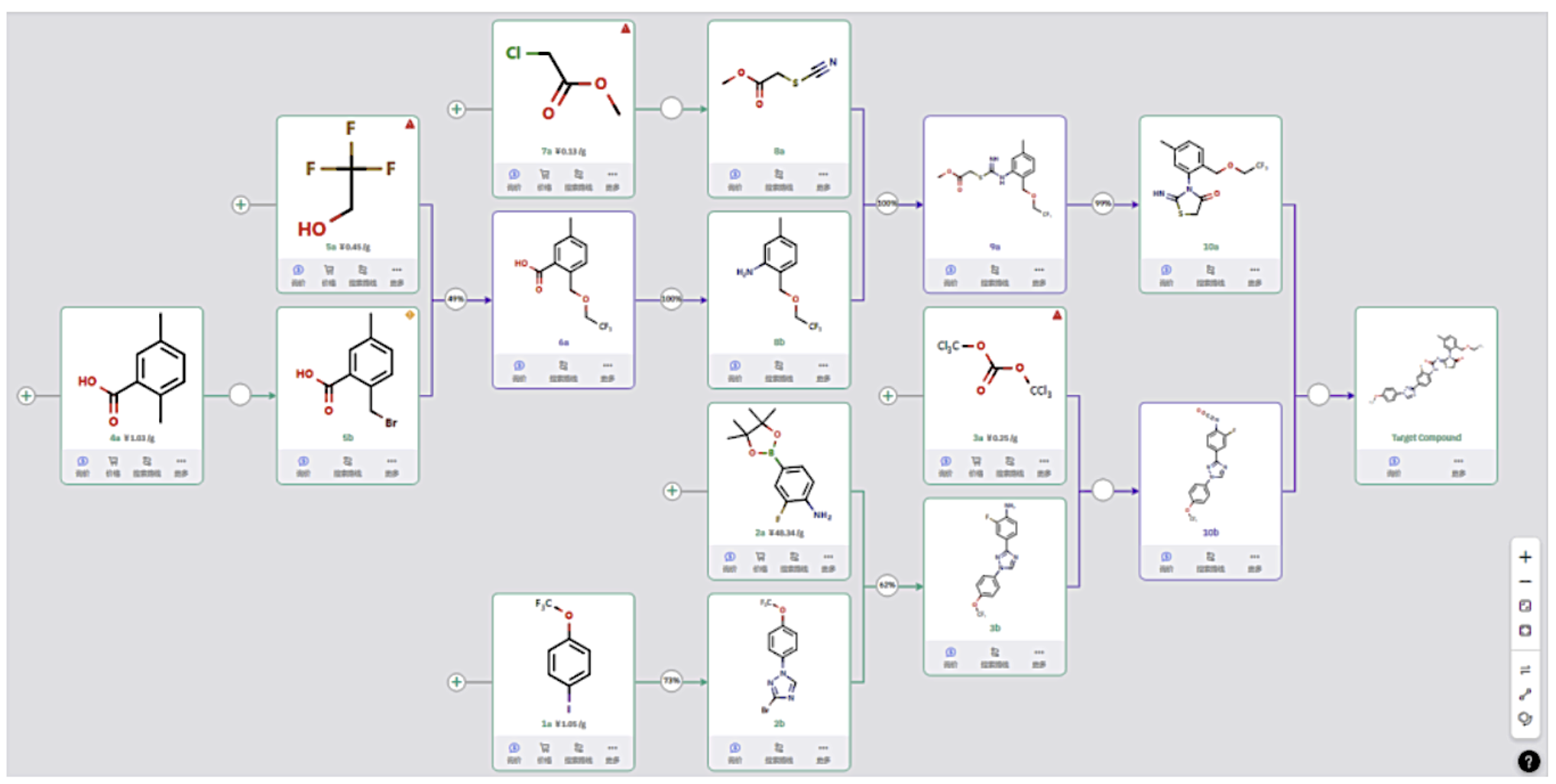
Route 1
Starting from simple raw material 4a, benzylic bromination, etherification, and conversion from carboxylic acid to amine produce intermediate 8b. Combined with thiocyanate compound 8a, this intermediate is converted in two steps into 10a.
In parallel, raw material 1a undergoes C–N bond formation to introduce a halogenated triazole unit, followed by Suzuki coupling to form a C–C bond. After isocyanate formation, coupling with compound 10a yields the final product.
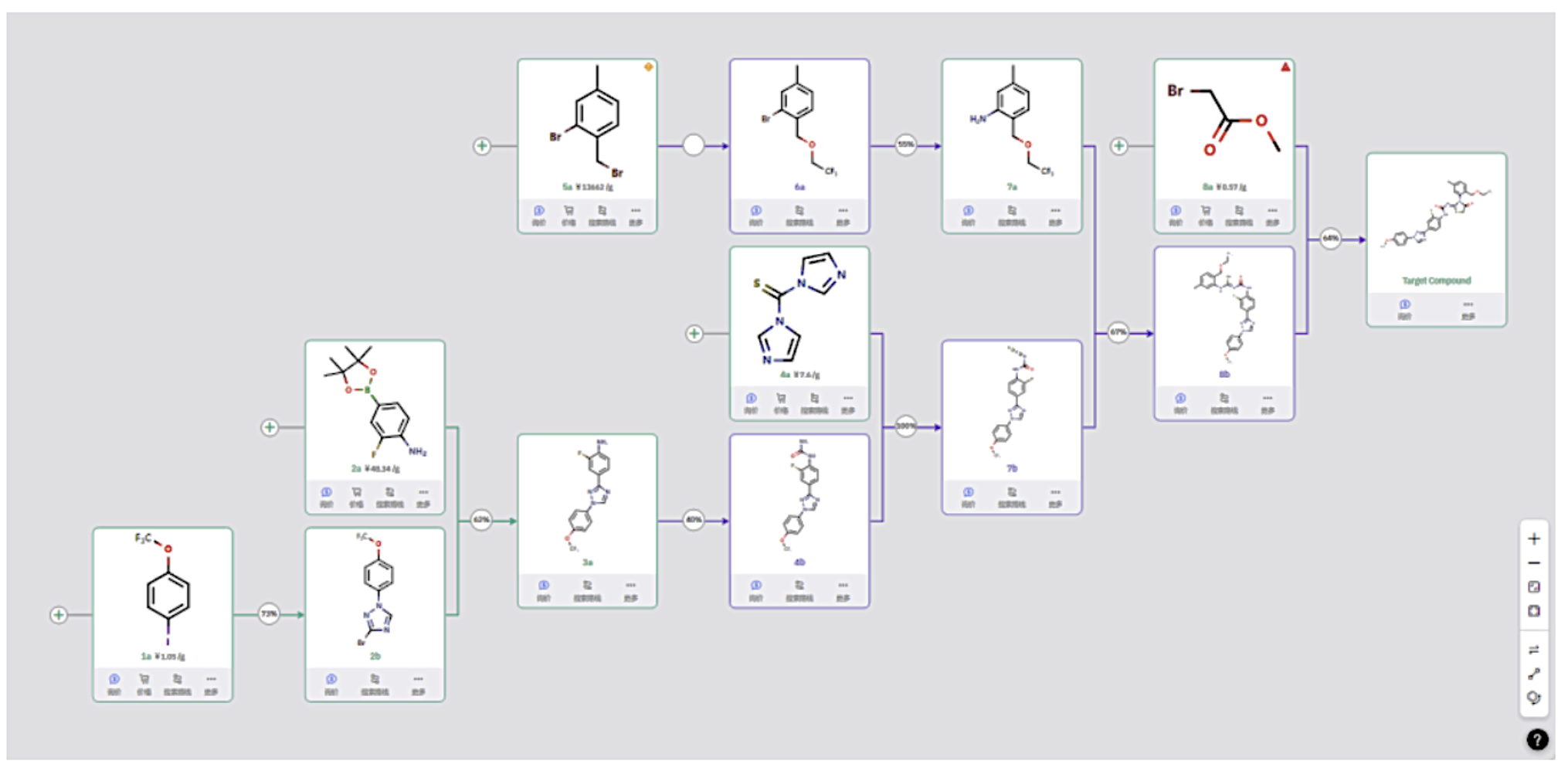
Route 2
Beginning with raw material 1a, a halogenated triazole is introduced via C–N bond formation, followed by Suzuki coupling. After building a urea structure, a thiocyanate group is introduced and reacted with amine intermediate 7a. Reaction with methyl bromoacetate then completes intermolecular substitution and ring closure to form the thiazoline ring.
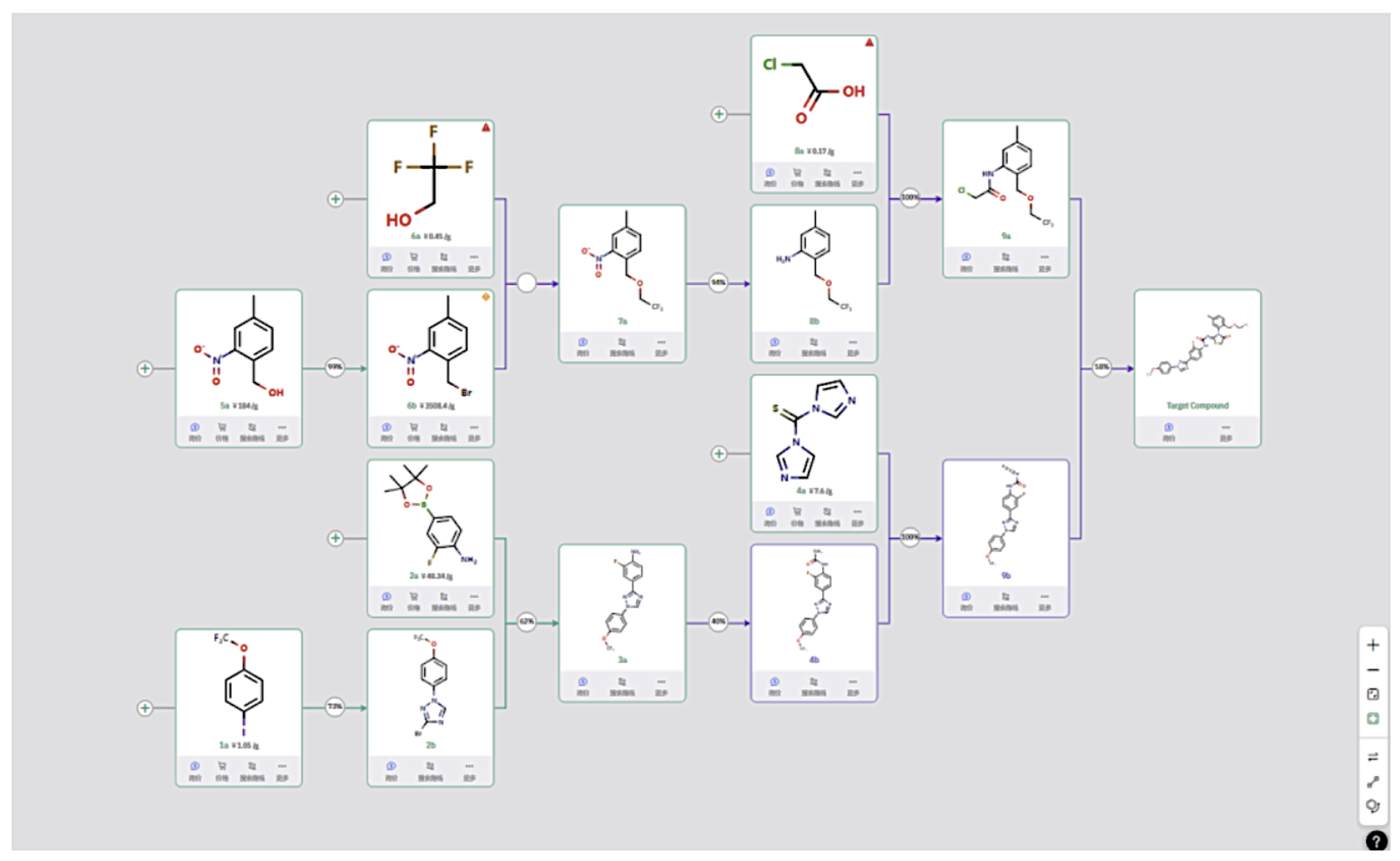
Route 3
This route follows a strategy similar to Route 2 but uses a different method to prepare intermediate 8b. In the ring-closing step, intermediates 9a and 9b undergo urea formation and intramolecular substitution to produce the final compound.
Industry Significance
Receiving an international common name usually signals that a pesticide is moving closer to commercialization. It suggests that early R&D is largely complete and that preparation for market entry has begun.
For the agrochemical industry, compounds like fentiazoluron with new modes of action are especially valuable - they are key tools for fighting pest resistance and updating integrated pest management strategies.
This matters even more as regulations tighten worldwide. For example, Mexico recently announced bans on 35 pesticides, including 2,4-DB, metolachlor, and chlorpyrifos-methyl. The industry urgently needs more selective, effective, and environmentally responsible alternatives. Fentiazoluron is expected to strengthen Corteva’s product portfolio while giving growers another option to protect crops and support food security.
With its ISO name now established, fentiazoluron has officially entered the global pesticide naming system. Attention will now turn to its mode of action, target pests, and real-world field performance - and to how it ultimately fits into modern agricultural practice.
ChemAIRS in Action
ChemAIRS Retrosynthesis generated practical routes to fentiazoluron, offering flexibility in both starting materials and synthetic strategy. This demonstrates ChemAIRS as a high-impact platform for accelerating complex molecule synthesis in agrichemistry workflows.
By allowing chemists to explore alternative pathways quickly, ChemAIRS enables faster experimentation, better decision-making, and stronger R&D outcomes.
Curious what ChemAIRS Retrosynthesis can do for your chemistry?
Learn more: ChemAIRS_Retrosynthesis